Medical degradable and absorbable Mg-Sr system magnesium alloy implant and preparation method thereof
A technology of implants and magnesium alloys, applied in prosthetics, medical science, coatings, etc., to achieve the effects of promoting tissue healing, inhibiting bone resorption, and promoting osteogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
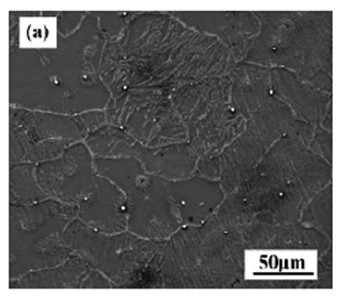
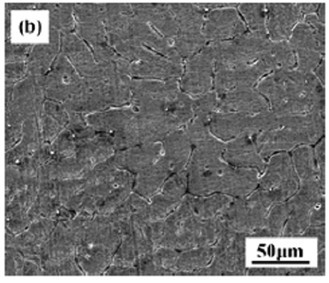
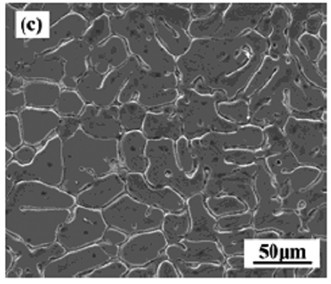
[0059] The test raw materials are pure Mg (99.95wt.%) and Mg-Sr master alloy, prepared according to Mg-xSr (nominal composition Sr: 1%-4%), in CO 2 +SF 6 Melting under the protection of atmosphere. First melt the pure magnesium ingot in a resistance furnace, and when the temperature rises to 750°C, add the Mg-Sr intermediate alloy. 2 +SF 6 Gravity casting is performed by pouring under the protection of mixed gas, and the mold is preheated to 200°C. Adopt the as-cast Mg-xSr alloy microstructure that the present invention obtains as figure 1 , figure 2 , image 3 , Figure 4 shown.
Embodiment 2
[0061] The alloy smelting process is the same as in Example 1. The Mg-xSr alloy ingot is processed into a 6mm thick plate, sanded until there are no obvious defects, and kept at 400°C for 3 hours before rolling. Furnace for 10 minutes, and finally rolled to a 1.2mm thick sheet. The as-rolled Mg-xSr alloy is processed into a tensile standard sample, and the tensile test at room temperature is carried out at a tensile speed of 1mm / min. The XRD spectrum of the as-rolled Mg-xSr alloy that adopts the present invention to obtain is as Figure 5 As shown, the room temperature tensile properties are as Figure 6 shown.
Embodiment 3
[0063] The as-rolled Mg-xSr alloy in embodiment 2 was prepared by wire cutting to 10×10×2mm 3 Sample piece, sanded with water sandpaper to 2000 # Afterwards, wash with acetone, absolute ethanol, and ultrasonic for 5 minutes each, dry, soak in Hank's simulated body fluid, and keep warm at 37°C. The samples were taken out after soaking for different time by X-ray diffraction (XRD) and scanning electron microscope (SEM) to detect the corrosion products on the alloy surface. The SEM morphology and XRD pattern of the alloy soaked for 10 days are as follows: Figure 7 , Figure 8 , Figure 9 , Figure 10 , Figure 11 , Figure 12 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com