Two-dimensional conjugated dibenzofuran conjugated polymer material and preparation method and application thereof
A technology of polymers and groups, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as low carrier mobility, low electrode collection efficiency, and mismatching radiation spectrum in the spectral response range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
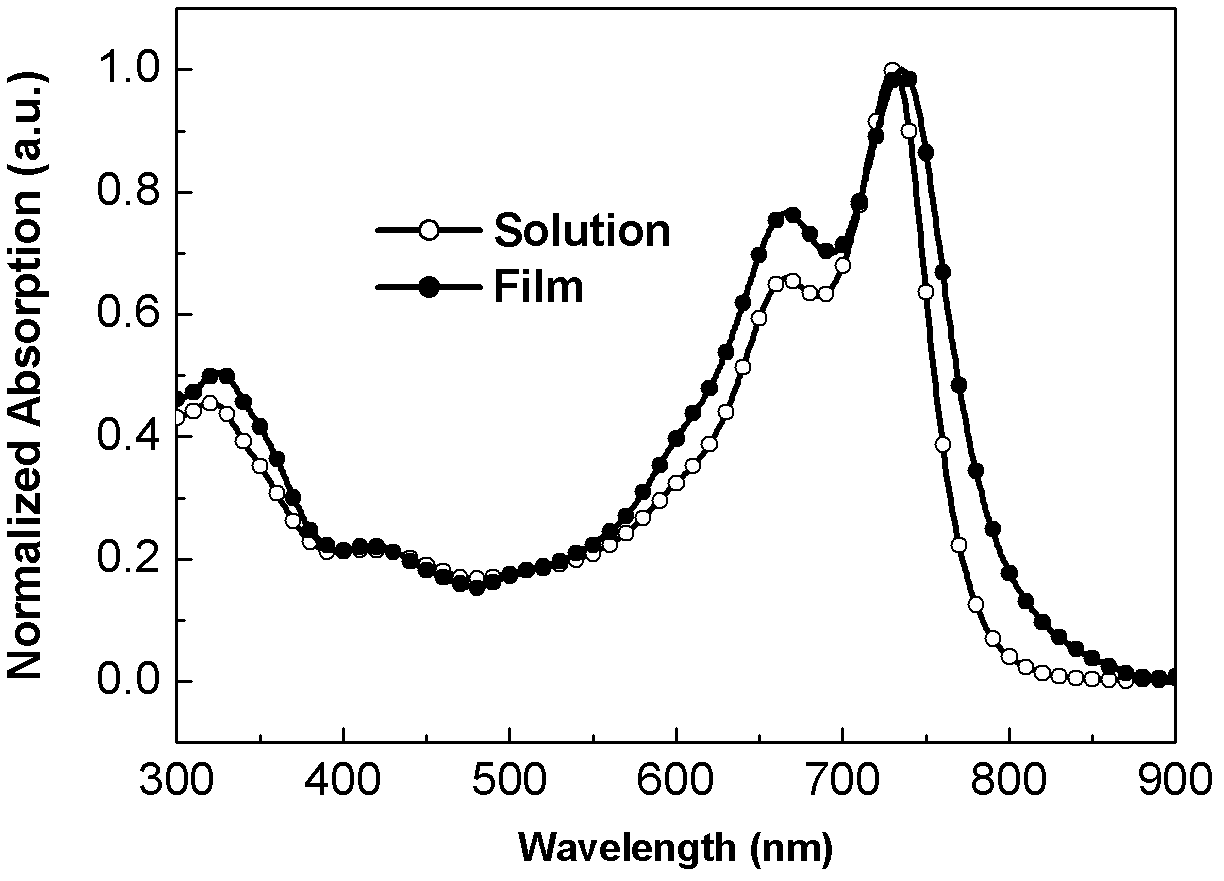
Image
Examples
Embodiment 1、2
[0072] Example 1, 2,6-bis(trimethyltin base)-4,8-bis(5-(2-ethylhexyl)thiophen-2 base)-benzo[1,2-b:4,5 Synthesis of -b']difuran
[0073] chemical reaction flow chart Figure 7 Shown, concrete reaction steps and reaction conditions are as follows:
[0074] N,N-Diacetamide-3-furan (compound 2, 28.47 g, 0.17 mol) synthesized according to the method disclosed by Keiji Kobayashi [J.Org.Chem.2000, 65, 2577-2579] was dissolved in anhydrous tetrahydrofuran (150 mL), n-butyl lithium (75 mL, 2.5 M) was added dropwise to the above reaction system in 5 minutes under an ice-water bath at 0° C., then slowly warmed to room temperature and kept stirring overnight. After 15 hours, dilute hydrochloric acid (100 mL, 3 mol / L) was added to the reaction system to quench the reaction. The organic phase of the mixture was extracted twice with dichloromethane, and the two extracted organic phases were combined and dried over anhydrous magnesium sulfate. After removal of the solvent, recrystallizati...
Embodiment 2
[0080] Example 2, poly{[4,8-bis(5-(2-ethylhexyl)thiophen-2 base)benzo[1,2-b:4,5-b']difuran-2,6- Synthesis of diyl]-co-[3-fluoro-thienyl[3,4-b]thiophen-2yl-]-2-ethylhexyl-1-one}(polymer PBDF-Th-TTCF).
[0081] chemical reaction flow chart Figure 8 Shown, concrete reaction steps and reaction conditions are as follows:
[0082] Take the monomer 2,6-bis(trimethyltinyl)-4,8-bis(5-(2-ethylhexyl)thiophen-2 base)-benzo[1,2-b:4,5- b']difuran (compound 5, 0.218g, 0.25mmol) and the monomer 4,6-dibromo-3-fluoro-thienyl[3,4-b]thiophen-2yl-]-2-ethylhexyl -1-ketone (TT-CF, 0.110g, 0.25mmol), dissolved in toluene (10mL), evacuated with argon for 5 minutes, then added the catalyst tetrakis(triphenylphosphine) palladium (0) (30mg ) and continue to exhaust the air for 25 minutes. Polymerization was then stopped at reflux temperature of toluene for 16 hours. The polymer solution was cooled to room temperature, slowly poured into methanol (50 mL), and the precipitated solid polymer was seque...
Embodiment 3
[0083]Example 3, poly{[4,8-bis(5-(2-ethylhexyl)thiophen-2 base)benzo[1,2-b:4,5-b']difuran-2,6- Synthesis of diyl]-co-5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione}(polymer PBDF-Th-TPD)
[0084] chemical reaction flow chart Figure 9 Shown, concrete reaction steps and reaction conditions are as follows:
[0085] Take the monomer 2,6-bis(trimethyltinyl)-4,8-bis(5-(2-ethylhexyl)thiophen-2 base)-benzo[1,2-b:4,5- b']difuran (compound 5, 0.218g, 0.25mmol) and the monomer 1,3-dibromo-5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4, 6(5H)-diketone (TPD, 0.105g, 0.25mmol), dissolved in a mixed solvent of toluene (8mL) and DMF (2mL), exhausted the air with argon for 5 minutes, and then added the catalyst tetrakis(triphenyl phosphine)palladium(0) (20 mg) and continued to evacuate the air for 25 minutes. Polymerization was then stopped after 15 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, slowly poured into methanol (50 mL), and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com