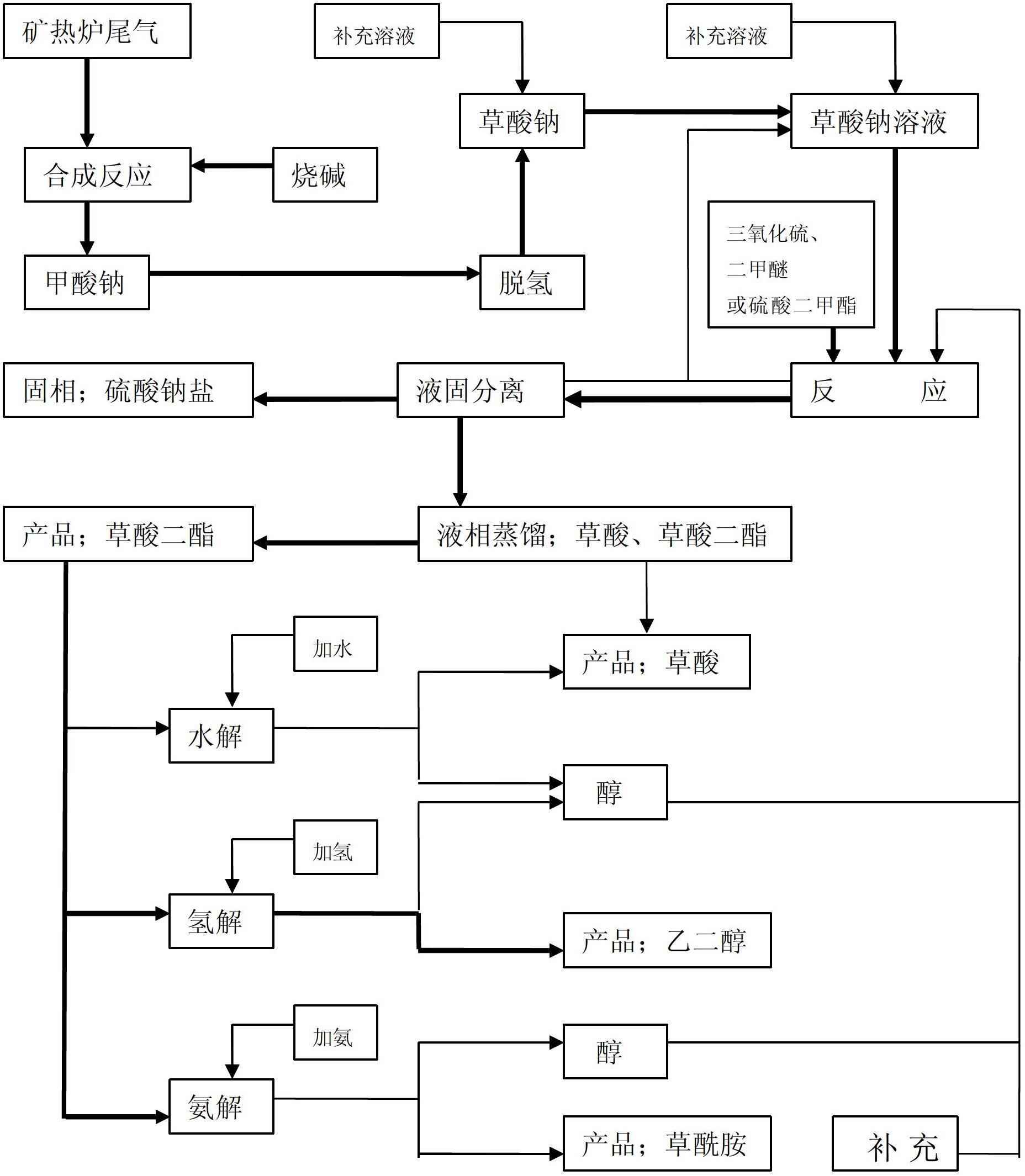
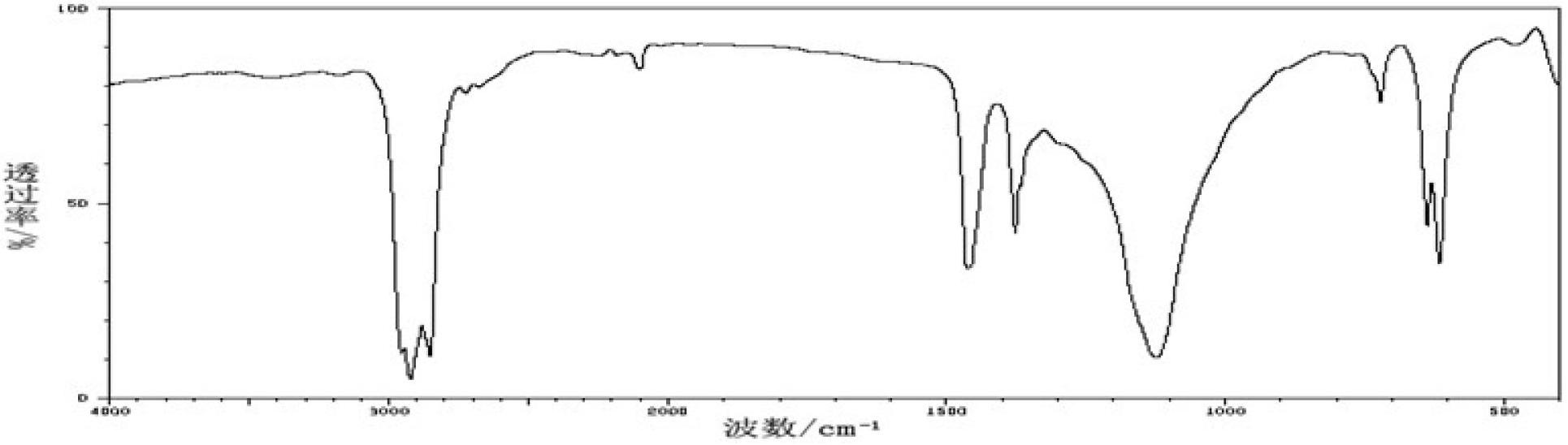
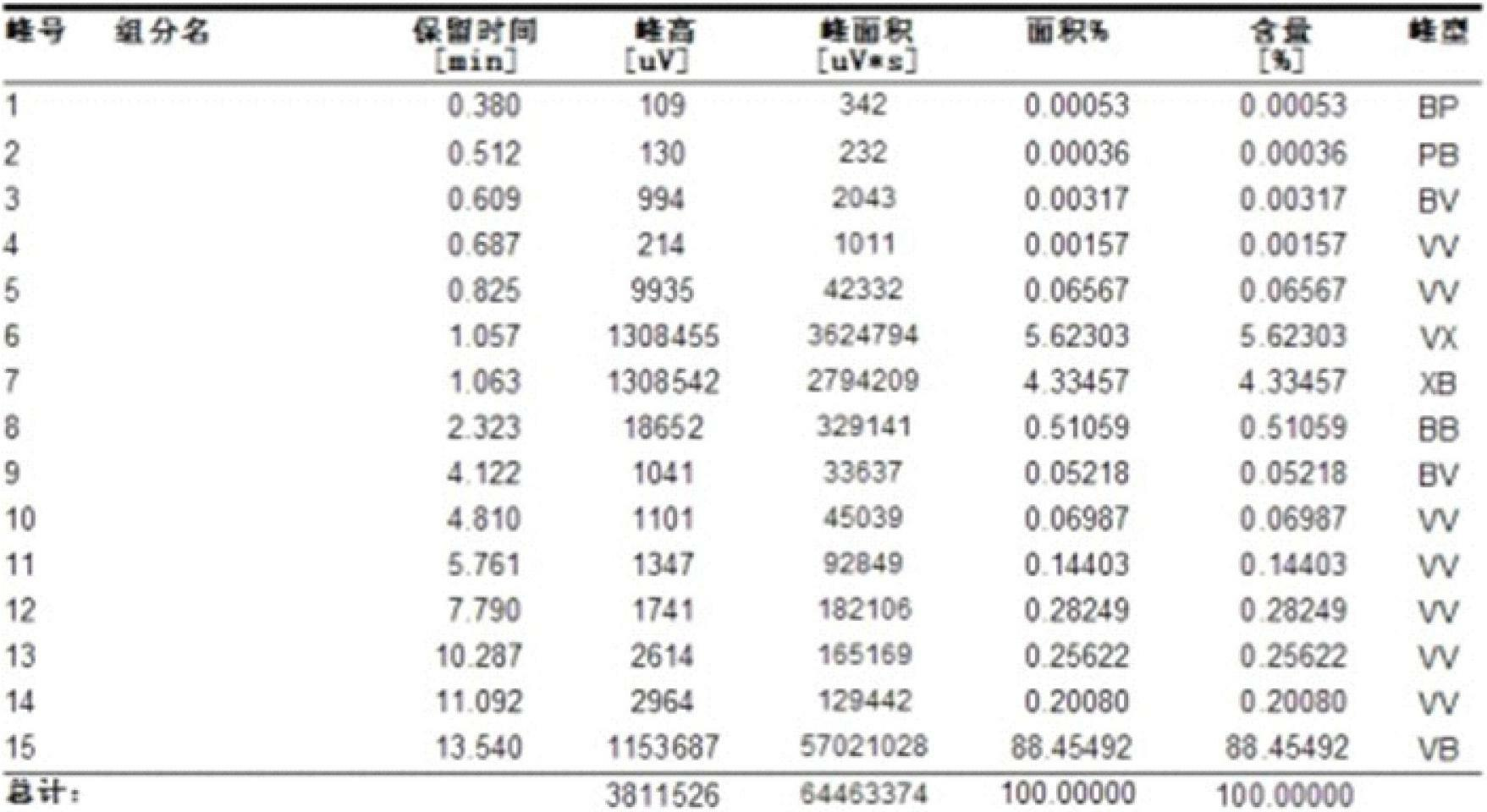
Chemical reaction system for producing oxalic acid diester as main products by using sodium formate as raw materials
An oxalic acid diester, chemical reaction technology, applied in the preparation of carboxylate, carboxylate/lactone, organic chemistry, etc., to achieve the effect of saving steam and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Sodium oxalate after dehydrogenation of sodium formate plus methanol as a solvent is made into a solution, and 15-20Kg of prepared sodium oxalate solution and 2-10Kg of recycled methanol are added into a stainless steel reaction kettle with a jacket, stirring, and condensation reflux device. Slowly add 7Kg of sulfur trioxide and 4Kg of dimethyl ether to react for 6 hours, stop stirring and settling for 2 hours, take out the upper clear liquid and set it as solution 1#, then take out the middle clear liquid and return it to sodium oxalate to prepare the solution. Then add 2-15Kg methanol and start stirring for 1 hour, stop stirring and settle for 2 hours, take out the supernatant liquid and set it as solution 2#, repeat the above method to obtain solution 4-6#, separate part of oxalic acid (trace amount) and dimethyl oxalate, Oxalic acid can be used as a product. Dimethyl oxalate is developed downstream by adding water to obtain oxalic acid, hydrogenation to obtain ethyl...
example 2
[0045] Sodium oxalate solution after dehydrogenation of sodium formate and solvent is made into a solution, and 1500Kg of prepared sodium oxalate solution and 200-1000Kg of recycled methanol are added into a stainless steel reaction kettle with a jacket, stirring, and condensation reflux device, and slowly put in Dimethyl sulfate 1100Kg reacts 8 hours, puts into airtight vacuum drum suction filter, solid phase is made into solution and enters next set of airtight vacuum drum suction filter, carries out airtight vacuum drum suction filter three times like this, solid The phase enters the vacuum distillation and drying equipment, and the high-temperature gas phase is condensed to obtain a liquid phase solution for reaction, and the sodium sulfate salt is dried after drying. The supernatant of the solution obtained by suction filtration for the first time is cooled to obtain crystallization of oxalic acid and dimethyl oxalate. After the supernatant is distilled, the residual liqui...
example 3
[0049] The solution is prepared by adding sodium oxalate after dehydrogenation of sodium formate and adding a solvent. Add 1500Kg of prepared sodium oxalate solution and 200-1000Kg of recycled methanol into a stainless steel reaction kettle with a jacket, stirring and condensing reflux device, and slowly put in React 650Kg of sulfur trioxide and 370Kg of dimethyl ether for 6 hours, stop stirring and settling for 3 hours, take out the clear night of the upper layer and set it as solution 1#, then take out the clear night of the middle layer and return it to sodium oxalate to prepare the solution. Then add 200-1500Kg methanol and start stirring for 0.5 hours, stop stirring and settle for 2 hours, take out the upper layer clear night and set it as solution 2#, then add 200-1500Kg methanol and start stirring for 0.5 hours, put it into a closed vacuum drum suction filter, and mix the solid phase The solution enters the next set of closed vacuum drum suction filter, and performs one ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com