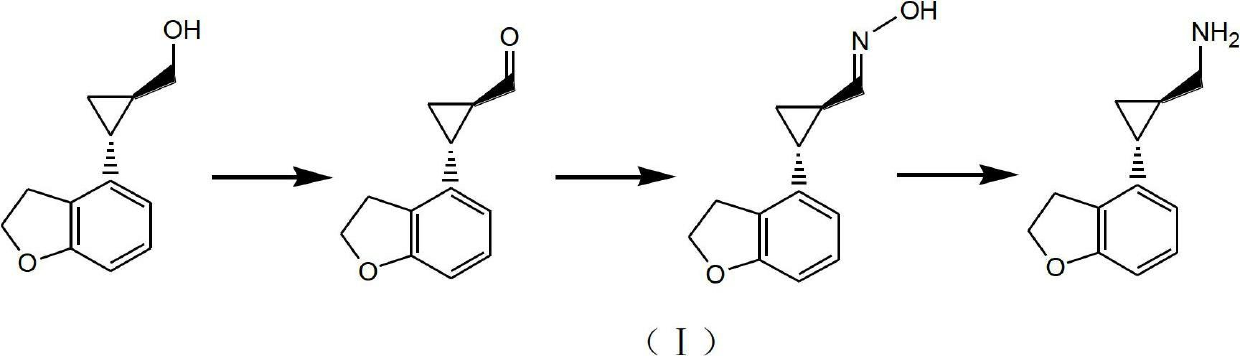
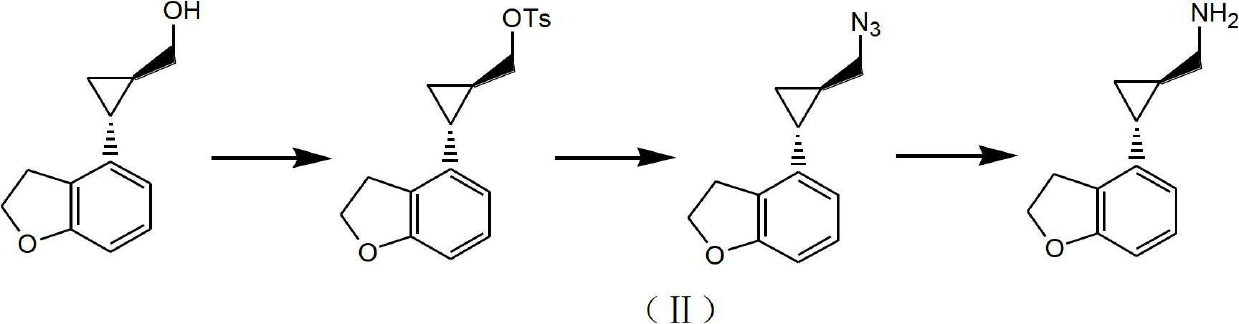
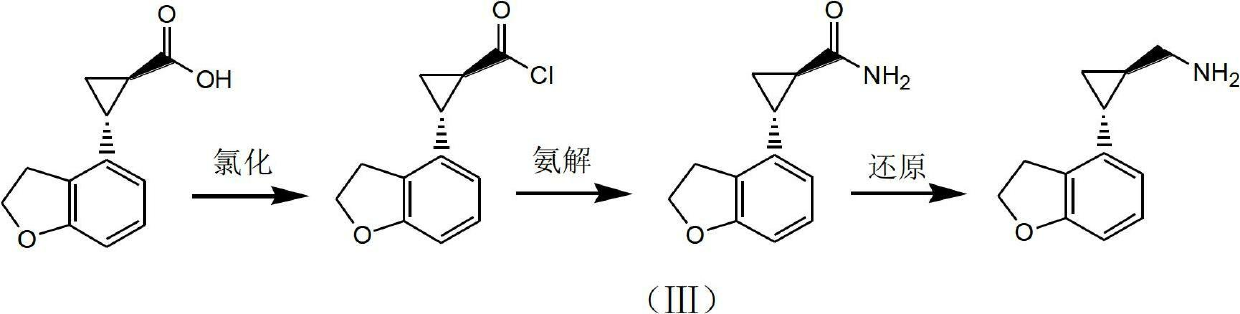
Method for preparing (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl) cyclopropanemethylamine
A technology of cyclopropyl methylamine and cyclopropyl carboxamide, which is applied in the field of key intermediates for preparing tasimelteon, can solve the problems of high risk of raw materials, harsh process conditions, difficult industrial scale-up and the like, and achieves high product purity and raw materials. Easy to obtain, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of tasimelteon
[0038] Proceed as follows:
[0039] (1) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarbonyl chloride
[0040] (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarboxylic acid 5.3g, dissolved in 100ml of dichloromethane, dropwise added thionyl chloride 12.4g, DMF0.02g, heated Reflux for 4 hours (the reflux temperature is the boiling point of the solvent methylene chloride, which is a conventional technique), take a sample and add methanol to oscillate, spot the plate to detect the disappearance of the raw materials and the reaction is complete. The solvent was distilled off with a rotary evaporator to obtain 5.8 g of oil, which was directly used in the next reaction.
[0041] (2) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropylcarboxamide
[0042] Add 5.8g of the oil obtained in the previous step to dissolve in 60ml of tetrahydrofuran, add dropwise 25g of ammonia water (mass concentrat...
Embodiment 2
[0047] Embodiment 2: the preparation of tasimelteon
[0048] (1) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarbonyl chloride
[0049] (1R,2R)-2-(2,3-Dihydrobenzofuran-4-yl)cyclopropanecarboxylic acid 4.3g, dissolved in 80ml of dichloromethane, dropwise added thionyl chloride 12.6g, DMF0.02g, room temperature Stir for 12 hours, take a sample and add methanol to oscillate, spot the plate to detect the disappearance of the raw materials and the reaction is complete. The solvent was distilled off with a rotary evaporator to obtain 4.6 g of oil, which was directly used in the next reaction.
[0050] (2) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropylcarboxamide
[0051] Add 50ml of tetrahydrofuran to 4.6g of the oil obtained in the previous step to dissolve, add dropwise 20g of ammonia water (mass concentration 19%), and stir at room temperature for 18 hours. Add 150ml of water, stir for 1 hour to precipitate a solid, filter, wash with wat...
Embodiment 3
[0056] Embodiment 3: the preparation of tasimelteon
[0057] (1) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarbonyl chloride
[0058] (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarboxylic acid 4.8g, dissolved in 96ml of methyl tert-butyl ether, added 4.0g of phosphorus oxychloride, heated to reflux for 6 After 1 hour, take a sample and add methanol to oscillate, and point the plate to detect the disappearance of the raw material and the reaction ends. The solvent was distilled off with a rotary evaporator to obtain 5.2 g of oil, which was directly used in the next reaction.
[0059] (2) Preparation of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropylcarboxamide
[0060] 5.2 g of the oil obtained in the previous step was dissolved in 52 ml of tetrahydrofuran, 22 g of aqueous ammonia (mass concentration 19%) was added dropwise, and stirred at room temperature for 18 hours. Add 150ml of water, stir for 1 hour to precipitate a solid, filter, wash w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com