System and method for expressing and purifying recombinant protein with 3'-nucleotidase as tag and application of recombinant protein
A nucleotidase and recombinant protein technology, applied in the field of recombinant protein expression and purification systems, can solve the problems of low protein purity, reduced sample loading speed, slow binding, etc. easy take off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
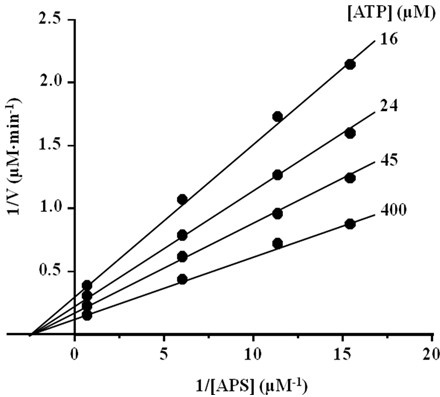
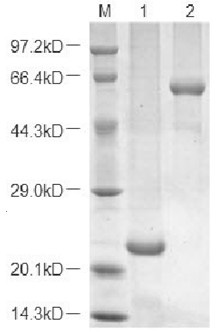
[0065] In this example, the protein expression and purification system was used to purify and analyze Escherichia coli APSK protein, and the kinetic analysis of the enzyme was carried out. The specific operation is as follows:
[0066] 1) Clone the yeast 3'-nucleotidase nucleotide sequence by PCR method (forward primer: GGAATTCCATATGCACCACCACCACCACGCATTGGAAAGAGAATTATTGG, reverse primer: AAGCTT CAGGGGCCCCTGGAACAGAACTTCCAG GGCGTTTCTTGACTGAATGAC, where the underline encodes the human rhinovirus 3C protease recognition sequence), and a human rhinovirus 3C protease recognition site was added to its C-terminus by PCR primer design.
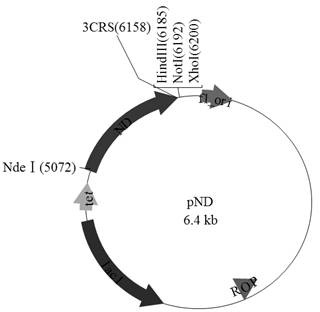
[0067] 2) The 3'-nucleotidase nucleotide sequence and pET24a were subjected to restriction endonuclease Nde I / Hind After III double digestion, use T4 ligase to connect and construct the expression vector pND, the subsequent Hind III, not I, xho IMultiple cloning sites can be used to insert target protein gene sequences. The expressed rec...
Embodiment 2
[0078] Such as Figure 12 As shown, the nucleotide sequence of crucian carp leptin protein (forward primer: AAGCTTTATTTTCCAGCTCTTCTCTACCCATG; reverse primer: CCGCTCGAGTTAGCAGCTTTTCAACTGGTCC) was cloned by PCR method and the expression vector pND was digested with restriction endonuclease Hind III / Xho I respectively, and then used The crucian carp leptin protein expression vector pND-CCL was constructed by T4 ligase ligation.
[0079] Other technical features of this embodiment are as described in Embodiment 1.
Embodiment 3
[0081] Such as Figure 13 As shown, the nucleotide sequence of Guanxi honey pomelo ζ-carotene desaturase (forward primer: GCGGCCGCATGGGTTTCTTCAGTTCTGTTTC; reverse primer: CCGCTCGAGTTAGTTCATCGTTAGTAGTAGTTG) nucleotide sequence and expression vector pND were cloned by PCR method, and the restriction enzymes Not I / Xho After I double enzyme digestion, T4 ligase was used to construct Guanxi honey pomelo ζ-carotene desaturase expression vector pND-CPZDS.
[0082] Other technical features of this embodiment are as described in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com