Preparation method of sulfonyl chloride compound taking carbazole as fluorogen
A technology of sulfonyl chlorides and compounds, which is applied in the field of synthesis of fluorescent molecular probes, can solve problems such as easy hydrolysis, insufficient detection sensitivity, and deviation of detection results, and achieve good material stability, reasonable process steps, and enhanced conjugation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
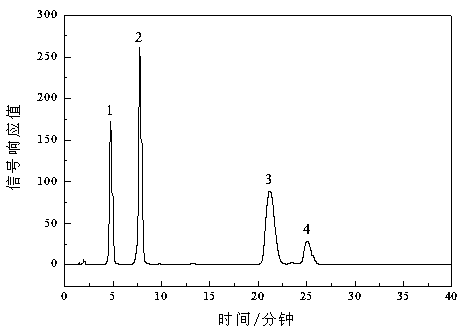
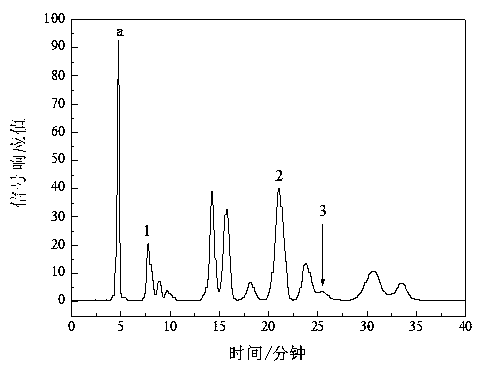
Image
Examples
Embodiment Construction
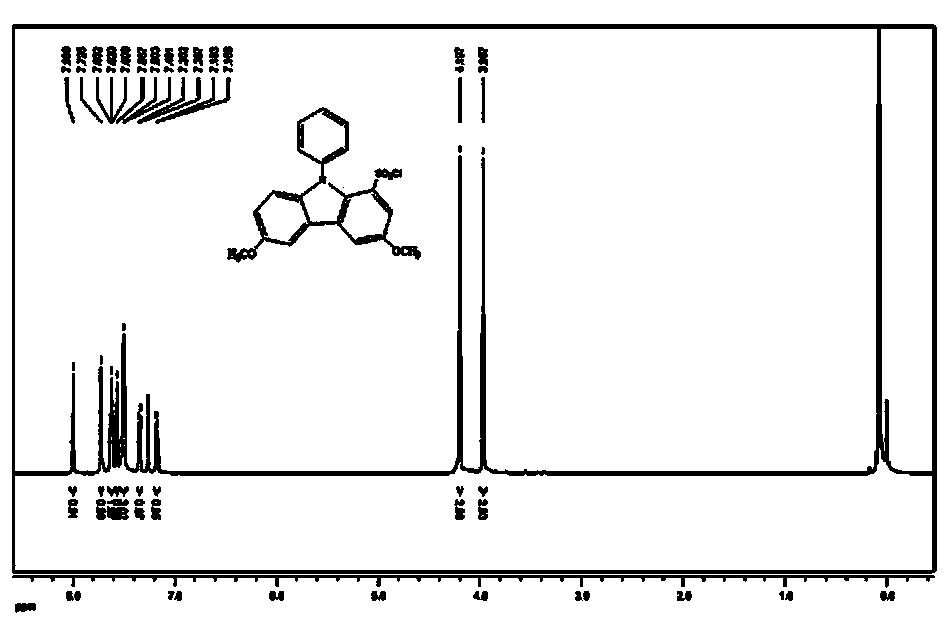
[0032] One, the synthetic steps of 3,6-dimethoxy-9-phenylcarbazole-1-sulfonyl chloride:
[0033] 1. Add 1.00 g carbazole, 16 g silica gel, 2.14 g N-bromosuccinimide and 50 mL dichloromethane into a 100 mL round bottom flask with magnetic stirring, and stir at room temperature for 10 hours. Then suction filter, purify, then add appropriate amount of distilled water and extract with ethyl acetate several times, and spin evaporate the solvent under reduced pressure, then recrystallize the solid with absolute ethanol, filter and dry to obtain 3,6-dibromocarbazole.
[0034] 2. Put a 100 mL round-bottomed flask with magnetic stirring in an ice-water bath, add 0.85 g of sodium to it after argon protection, and slowly drop 10 mL of anhydrous methanol into it. When the metal sodium is completely dissolved to form sodium methoxide, Then add 0.6 g of 3,6-dibromocarbazole, 1.48 g of cuprous iodide, 20 mL of anhydrous N,N-dimethylformamide, heat and reflux for 4 hours, stop the heating, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com