Dexketoprofen tromethamine sustained release particles and preparation method and sustained release medicinal preparation thereof
A technology of dexketoprofen tromethamine and sustained-release microparticles, applied in the field of medicine, can solve the problems of large side effects of dexketoprofen tromethamine, ulcers or bleeding symptoms, etc. Small concentration, the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
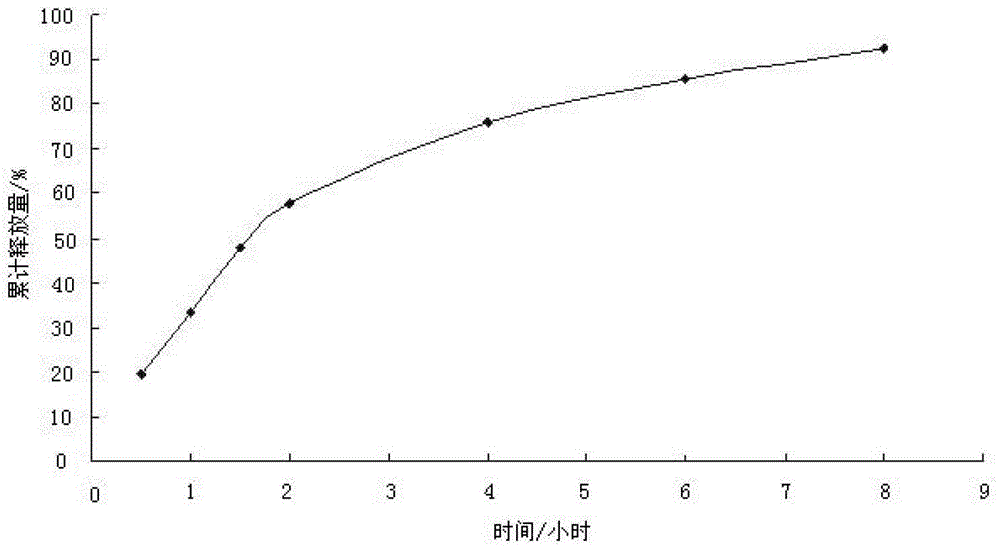
[0034] The dexketoprofen trometamol slow-release microparticles of the present embodiment are made by coating the dexketoprofen trometamol microparticles with a coating solution; the dexketoprofen trometamol microparticles are combined with The mass ratio of coating liquid is 1: 1; Described dexketoprofen tromethamine microparticle is made of the raw material of following percentage by weight: dexketoprofen tromethamine 10%, diluent 60%, stable Agent 10%, binding agent 20%; Described coating solution is made of the raw material of following percentage by weight: coating material 2%, porogen 5%, solvent 93%; Described diluent is microcrystalline cellulose; The stabilizer is sodium bisulfite; the binder is water; the coating material is ethyl cellulose; the porogen is polyvinylpyrrolidone; the solvent is ethanol with a mass purity of 95% .
[0035] The preparation method of the dexketoprofen trometamol slow-release microparticles of the present embodiment is:
[0036] Step 1, pr...
Embodiment 2
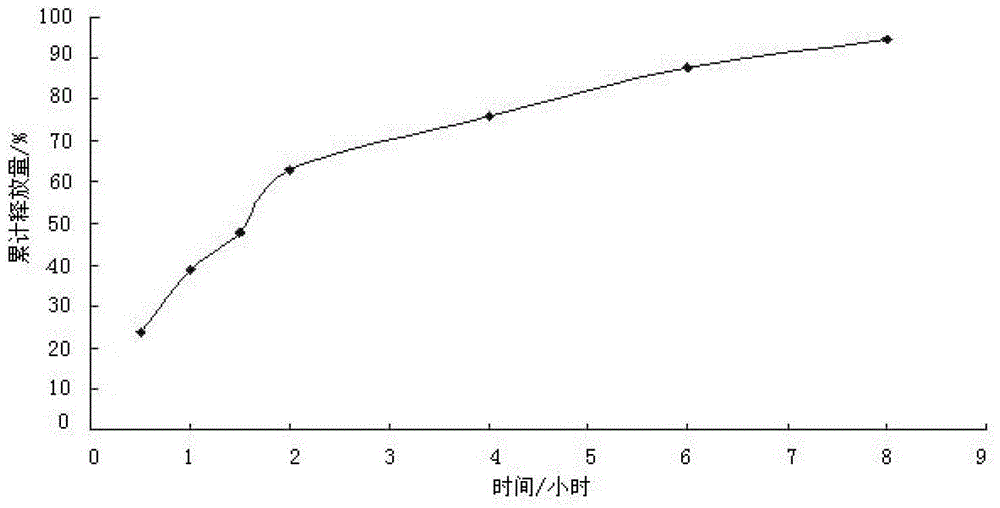
[0045] This embodiment is the same as Example 1, except that the diluent is lactose, starch or mannitol, or at least two of microcrystalline cellulose, lactose, starch and mannitol; the stabilizer Cystine, cysteine or hydrochloric acid with a concentration of 0.5mol / L to 2mol / L, or cystine, cysteine, sodium bisulfite and hydrochloric acid with a concentration of 0.5mol / L to 2mol / L At least two kinds of hydrochloric acid; the binder is a hydroxypropyl methylcellulose aqueous solution with a mass concentration of 0.5% to 8%, an aqueous starch solution with a mass concentration of 1% to 10%, or a starch aqueous solution with a mass concentration of 1% to 10% % dextrin aqueous solution; the coating material is cellulose acetate, cellulose acetate derivatives (such as: cellulose acetate phthalate (CAP), cellulose acetate tetrahydrophthalate (CATH ) and cellulose acetate methyltetrahydrophthalate (CAMT), etc.), polyacrylic resin, shellac or waxes (such as: glyceryl monostearate, e...
Embodiment 3
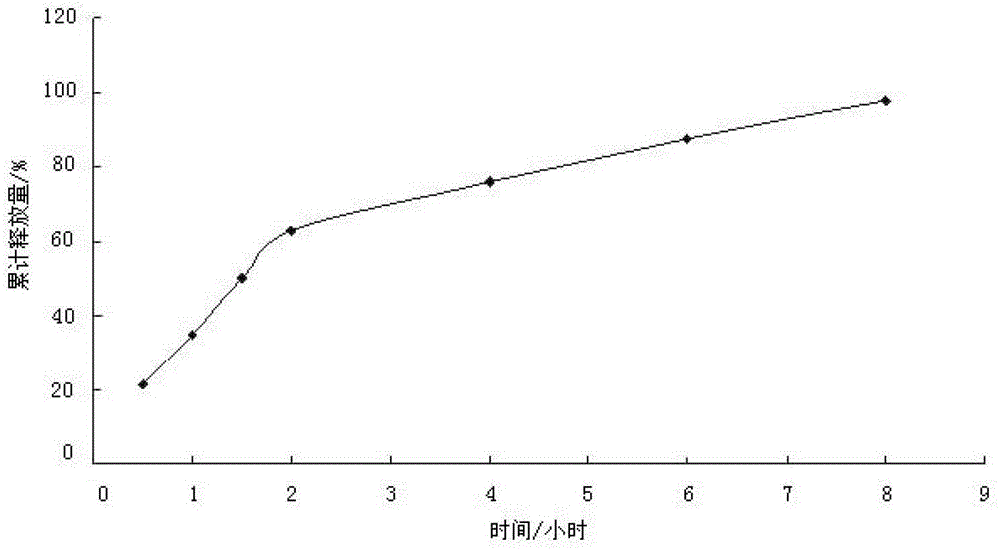
[0047] The dexketoprofen trometamol slow-release microparticles of the present embodiment are made by coating the dexketoprofen trometamol microparticles with a coating solution; the dexketoprofen trometamol microparticles are combined with The mass ratio of coating liquid is 4: 1; Described dexketoprofen tromethamine microparticle is made of the raw material of following percentage by weight: dexketoprofen tromethamine 30%, diluent 35%, stable Agent 5%, binding agent 30%; Described coating liquid is made of the raw material of following percentage by weight: coating material 10%, porogen 1%, plasticizer 2%, solvent 87%; Described diluent It is microcrystalline cellulose and starch; the stabilizer is cysteine; the binder is water; the coating material is polyacrylic acid resin; the porogen is polyethylene glycol; The plasticizer is glycerol triacetate; the solvent is water.
[0048] The preparation method of the dexketoprofen trometamol slow-release microparticles of the pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com