Polymer monolithic column, and preparation method and application thereof
A polymer, monolithic column technology, applied in the field of liquid chromatography, to achieve the effect of many action sites, easy operation and low column back pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Glycidyl methacrylate, ethylene glycol dimethacrylate, cyclohexanol, dodecanol, and azobisisobutyronitrile are respectively 18.0%, 17.6%, 56.5%, 6.9%, and 1.0% by weight Mixed, purged with nitrogen to remove oxygen, ultrasonicated for 10 minutes, poured into a stainless steel column tube with a syringe, sealed at both ends, and polymerized in a water bath at 60°C for 24 hours. The polymerized column was flushed with 100 mL of methanol into the porogen to obtain a blank chromatographic monolithic column. Normal experiments were performed after the cartridge had equilibrated for one hour with the mobile phase running.
Embodiment 2
[0035] Glycidyl methacrylate, ethylene glycol dimethacrylate, cyclohexanol, dodecanol, and azobisisobutyronitrile are respectively 18.0%, 17.6%, 56.5%, 6.9%, and 1.0% by weight Mixed, purged with nitrogen to remove oxygen, ultrasonicated for 10 minutes, poured into a stainless steel column tube with a syringe, sealed at both ends, and polymerized in a water bath at 60°C for 24 hours. The polymerized column was flushed with 100 mL of methanol as a porogen, and then the epoxy group on the surface of the whole column was hydrolyzed with 0.25 M dilute sulfuric acid at 60°C for 4 hours. 40 mL of an aqueous solution of 1-butyl-3-vinylimidazolium chloride was passed through the column for 13 hours, and the temperature was controlled at 80°C. The grafted column body is washed with water to obtain a chromatographic monolithic column with a mixed separation mode. Finally the column was equilibrated with the mobile phase running for one hour for normal experiments.
Embodiment 3
[0036] Embodiment 3, separation experiment:
[0037] 1
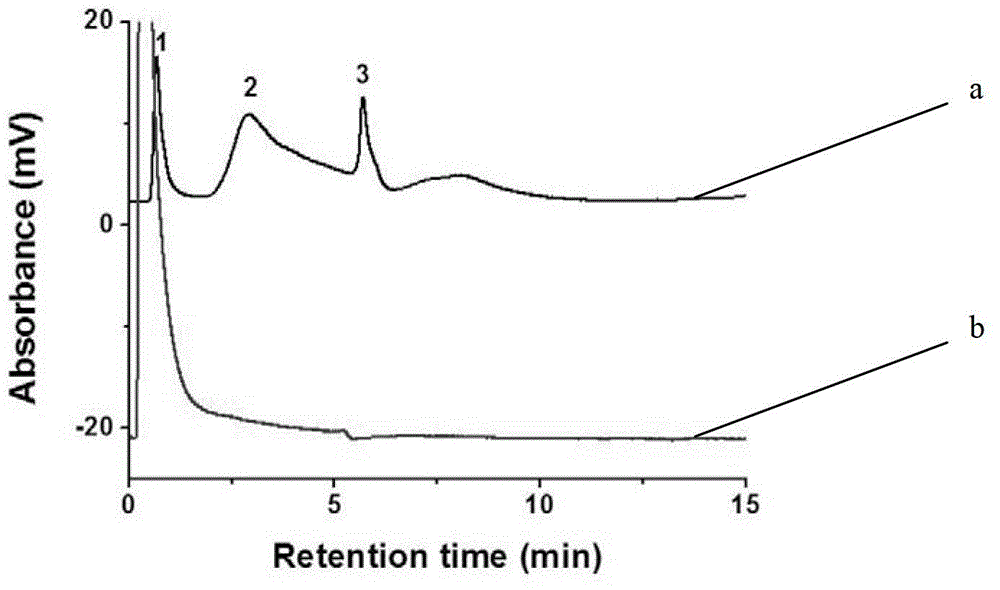
[0038] Using the monolithic column in Example 2 as the stationary phase, VB1, VB3, and VB9 were separated in anion exchange mode. Use 20.0mM pH7.0 phosphate buffer as mobile phase A, the above phosphate buffer containing 1.0M sodium chloride as mobile phase B, gradient elution: 0 minutes, 3% B; 4.49 minutes, 3% B; 4.50 minutes , 50%B; flow rate, 1.0mL / min; UV detection wavelength, 254nm.
[0039] The obtained chromatogram is as figure 1 As shown in the curve a, it can be seen from the figure that the three vitamin B substances have been basically separated, and the order of the peaks is: VB1, VB3, and VB9.
[0040] 2
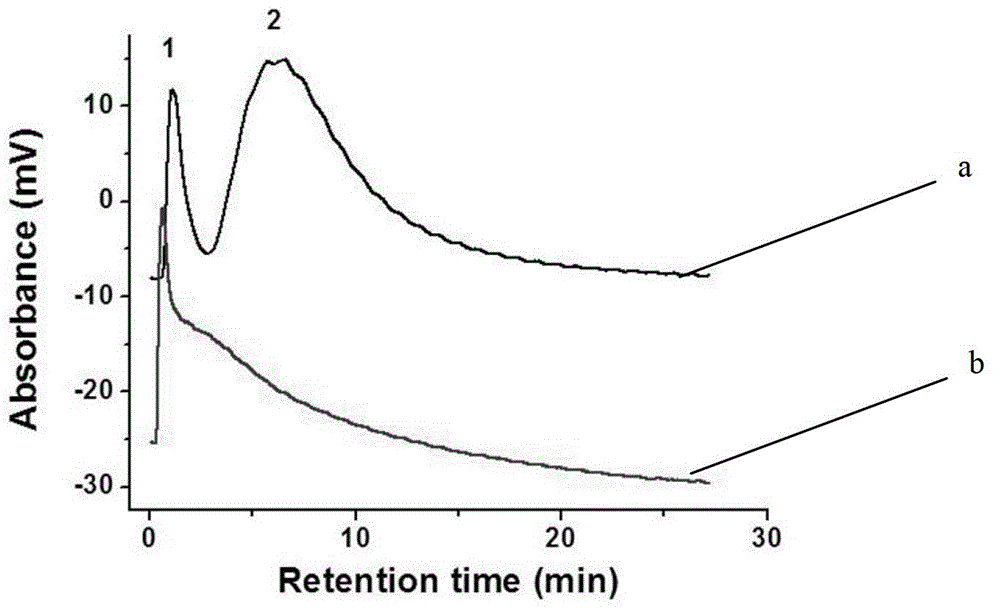
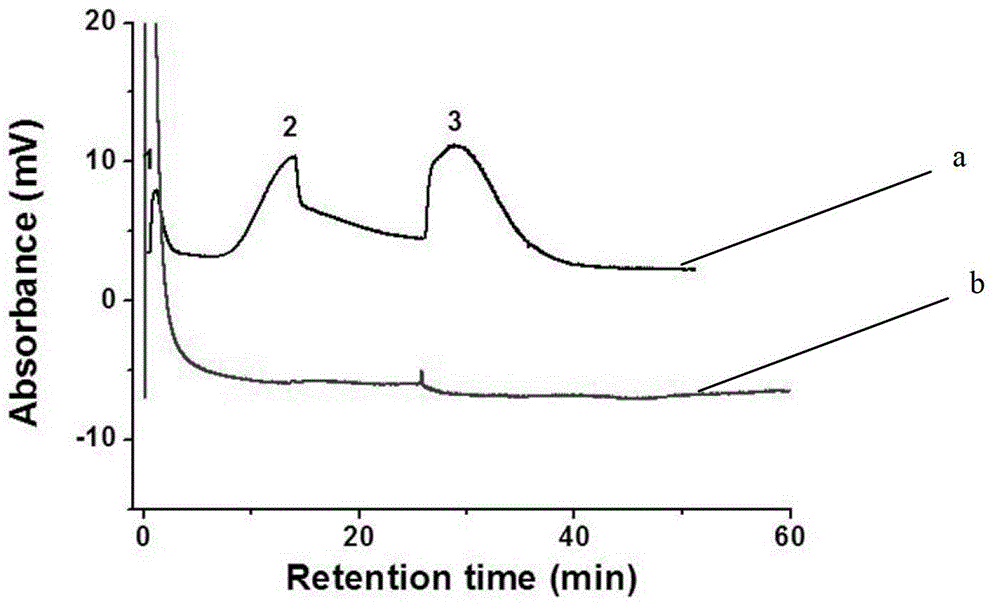
[0041] Using the monolithic column in Example 1 as the stationary phase, VB1, VB3, and VB9 were separated in anion exchange mode. Use 20.0mM pH7.0 phosphate buffer as mobile phase A, the above phosphate buffer containing 1.0M sodium chloride as mobile phase B, gradient elution: 0 minutes, 3% B; 4.49 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com