High-efficiency hydrogenation catalyst and method for preparing same
A hydrogenation catalyst and catalyst technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of increasing catalyst preparation or production costs, high active metals and auxiliary metals, and achieve high resistance to sintering Effects of deactivation ability, high catalytic activity and life, and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of efficient hydrogenation catalyst
[0042] Weigh 72g of copper nitrate and 0.7g of lanthanum nitrate and dissolve them in deionized water to prepare an aqueous solution with an ion concentration of 0.3M. Weigh 45.5g of urea and dissolve it in deionized water to prepare an aqueous solution with a concentration of 0.6M. Weigh 230g of the silica sol solution and adjust the pH value between 1-4 with nitric acid. Add the above-mentioned metal ion solution and urea solution into the silica sol solution (the order of adding the two is in no particular order), and stir to obtain a mixed solution. Transfer the mixed solution into a reaction flask, stir for 0.5-2.5 hours, then raise the temperature to 60-105° C. for reaction, the heating time is 0.8-1.2 hours, and the reaction time is 24 hours. After the reaction is completed, vacuum filter while it is hot, wash with deionized water and methanol or ethanol, and dry at 120°C for 12 hours. After d...
Embodiment 2
[0047] Embodiment 2: the preparation of efficient hydrogenation catalyst
[0048] Weigh 72g of copper nitrate and 14.5g of nickel nitrate and dissolve them in deionized water to form an aqueous solution with a total ion concentration of 0.3M. Weigh 45.5g of urea and dissolve it in deionized water to prepare an aqueous solution with a concentration of 0.6M. Weigh 230g of the silica sol solution and adjust the pH value between 1-4 with nitric acid.
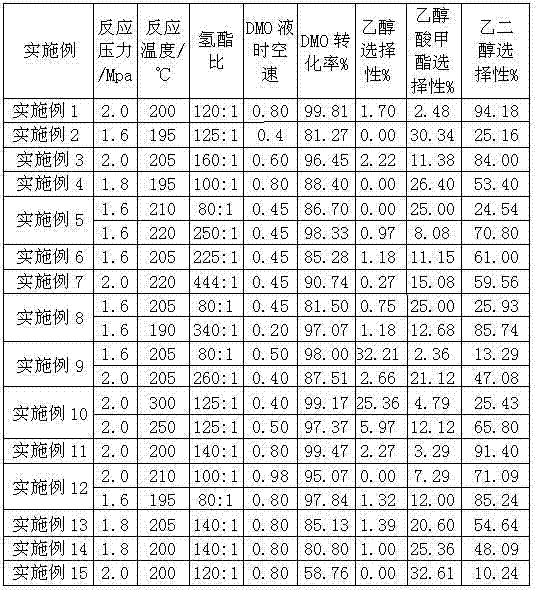
[0049] The rest of the preparation process was the same as in Example 1, and the prepared catalyst had an active component content of 25%, an additive metal component content of 5%, and a carrier content of 70%. See attached table 1 for reaction process conditions and reaction results.
[0050] The catalyst has a relatively high conversion rate of dimethyl oxalate and selectivity of methyl glycolate, and the process is stable without obvious deactivation after running in a fixed-bed reactor for 300 hours.
Embodiment 3-5
[0051] Embodiment 3-5: Preparation of efficient hydrogenation catalyst
[0052] Others are identical with embodiment 2, by changing the add-on of nickel nitrate, be respectively 2.9g, 1.45g, 43.5g. See attached table 1 for reaction process conditions and reaction results.
[0053] The prepared catalyst has an active metal component content of 25%, a promoter metal component content of 0.5%, 1%, and 15%, and a carrier content of 74.5%, 74%, and 60%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com