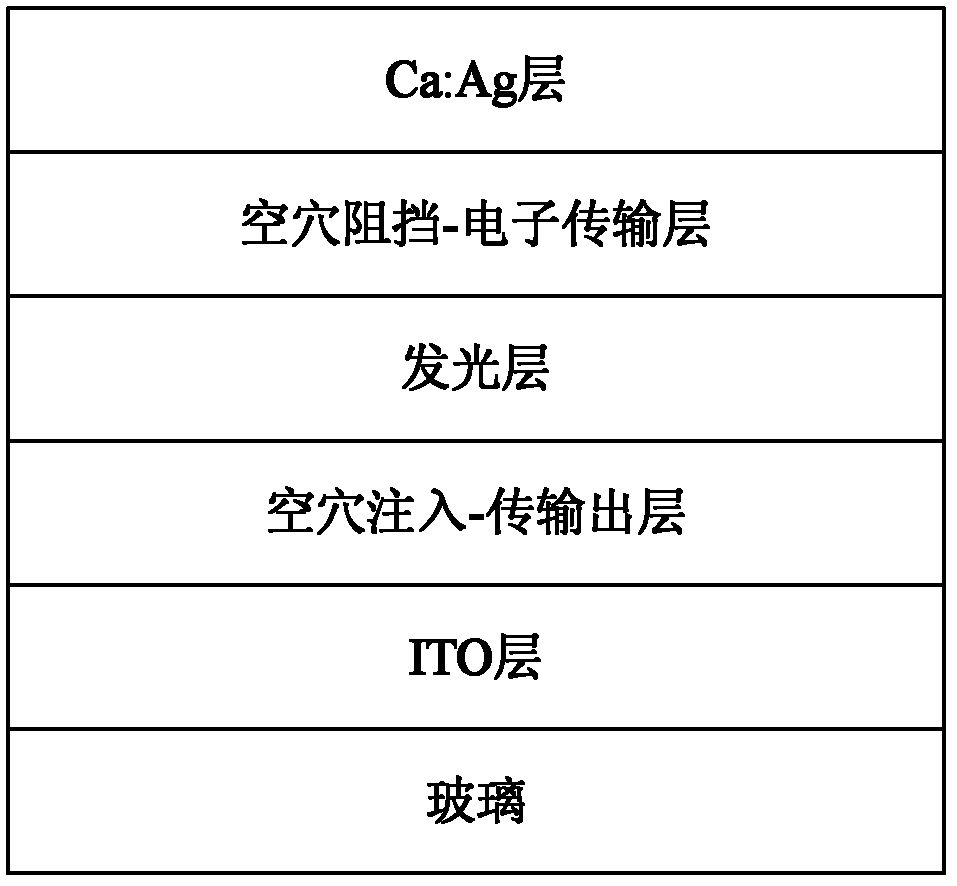
Cyano anthraquinone derivatives, preparation method and organic electroluminescent devices thereof
A technology of cyanoanthraquinone and tetracyanoanthraquinone is applied in the field of organic optoelectronic materials, which can solve the problems of inability to form high-quality thin films, device failure, and rough surface, and achieve good morphology stability and high singlet energy. The effect of grade and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
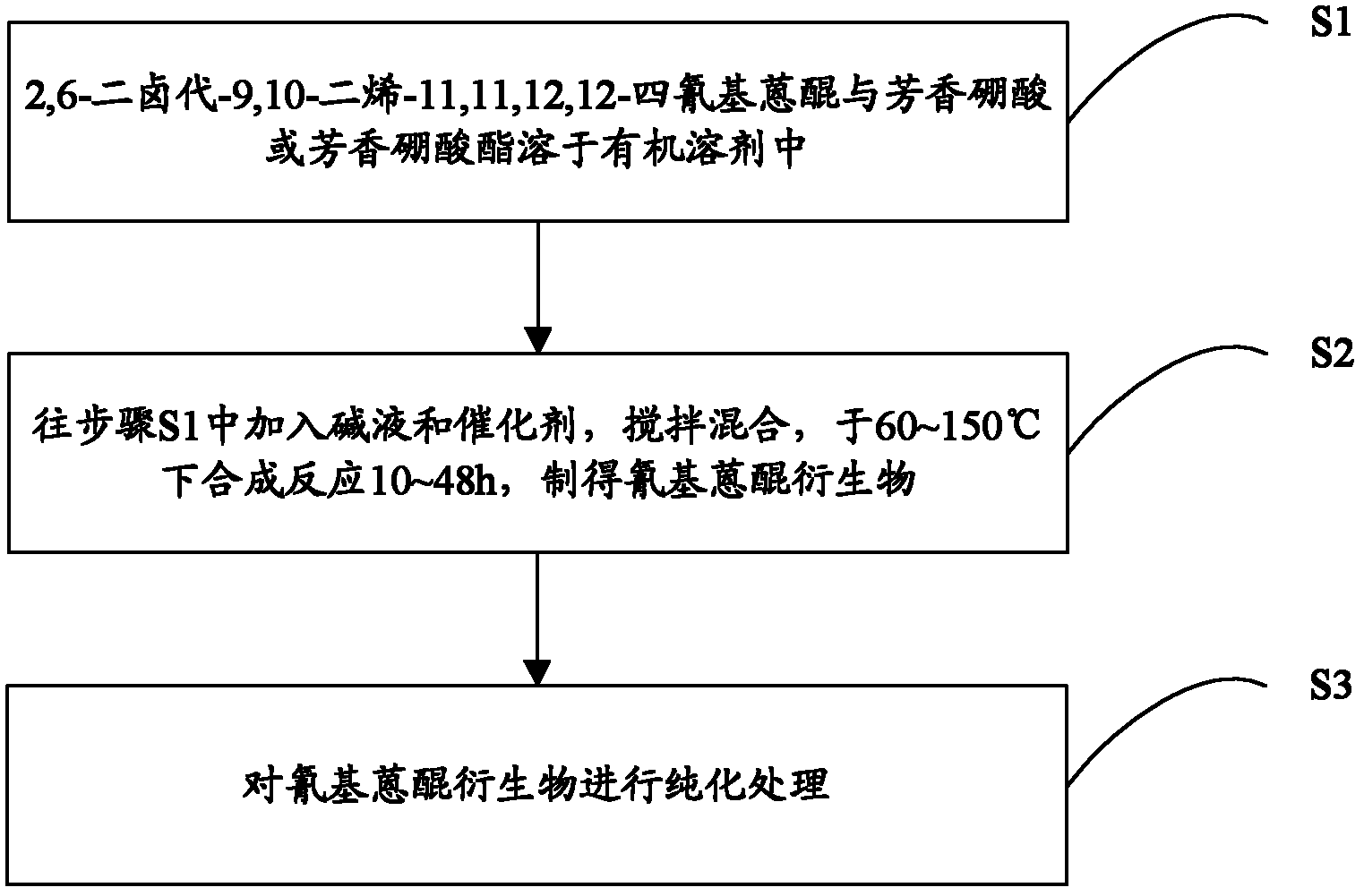
[0021] Another object of the present invention is to provide a preparation method of cyanoanthraquinone derivatives, which includes the following steps:
[0022] Step S1, the structural formula is The 2,6-dihalo-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the structural formula The aromatic boric acid or structural formula is The aromatic borate is soluble in organic solvents; among them, Ar is an aromatic group; X is Cl or Br; 2,6-dihalo-9,10-diene-11,11,12,12-tetracyano The molar ratio of anthraquinone to aromatic boric acid or aromatic boric acid ester is 1:2-1:4;
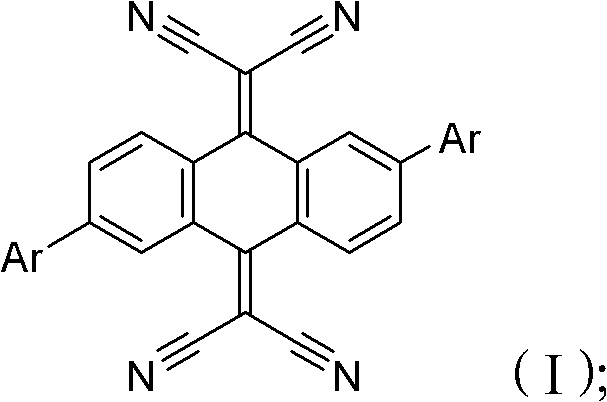
[0023] Step S2, adding lye and catalyst to step S1, stirring and mixing, and synthesizing reaction at 60-130°C for 10 to 48 hours to obtain the cyanoanthraquinone derivative of the following general formula (I):
[0024]
[0025] The reaction formula is as follows:
[0026]
[0027] The further optimization scheme of the above preparation method is that the crude product of the cyanoanthraquinone derivative o...
Embodiment 1
[0041] This example discloses 2,6-dipyrene-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the following structural formula.
[0042]
[0043] The preparation of the above target molecule is as follows:
[0044] Under the protection of nitrogen, 2,6-dibromo-9,10-diene-11,11,12,12-tetracyanoanthraquinone (4.15g, 9mmol), pyreneboric acid (5.76g, 23.4mmol) and tetra Triphenylphosphine palladium (104 mg, 0.09 mmol) was dissolved in 80 ml of ethylene glycol dimethyl ether, then sodium carbonate solution (2.0 mol / L, 90 mL) was added and mixed; the mixture was stirred vigorously at 90° C. for 12 hours. Cooled to room temperature, extracted three times with dichloromethane, combined the organic phases, washed with 1mol / L sodium hydroxide solution, dried over anhydrous magnesium sulfate and spin-dried. The crude product was petroleum ether: ethyl acetate (10:1) as The eluent is separated by silica gel chromatography to obtain a white solid, namely 2,6-dipyrene-9,10-diene-11,11,12,12-te...
Embodiment 2
[0047] This example discloses 2,6-bis[10'-(9'-phenyl)anthracene]-9,10-diene-11,11,12,12-tetracyanoanthraquinone with the following structural formula.
[0048]
[0049] The preparation of the above target molecule is as follows:
[0050] Under the protection of nitrogen, 2,6-dibromo-9,10-diene-11,11,12,12-tetracyanoanthraquinone (4.15g, 9mmol), 9-phenyl-10-anthraboronic acid (5.76 g, 23.4mmol) and bistriphenylphosphonium palladium dichloride (315.9mg, 0.45mmol) were dissolved in 80ml of tetrahydrofuran, and then sodium carbonate solution (2.0mol / L, 90mL) was added. The mixture was stirred vigorously at 90°C for 18h. Cooled to room temperature, extracted three times with dichloromethane, combined the organic phases, washed with 1mol / L sodium hydroxide solution, dried over anhydrous magnesium sulfate and spin-dried. The crude product was petroleum ether: ethyl acetate (10:1) as The eluent is separated by silica gel chromatography to obtain a white solid, namely 2,6-bis[10'-(9'-phen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com