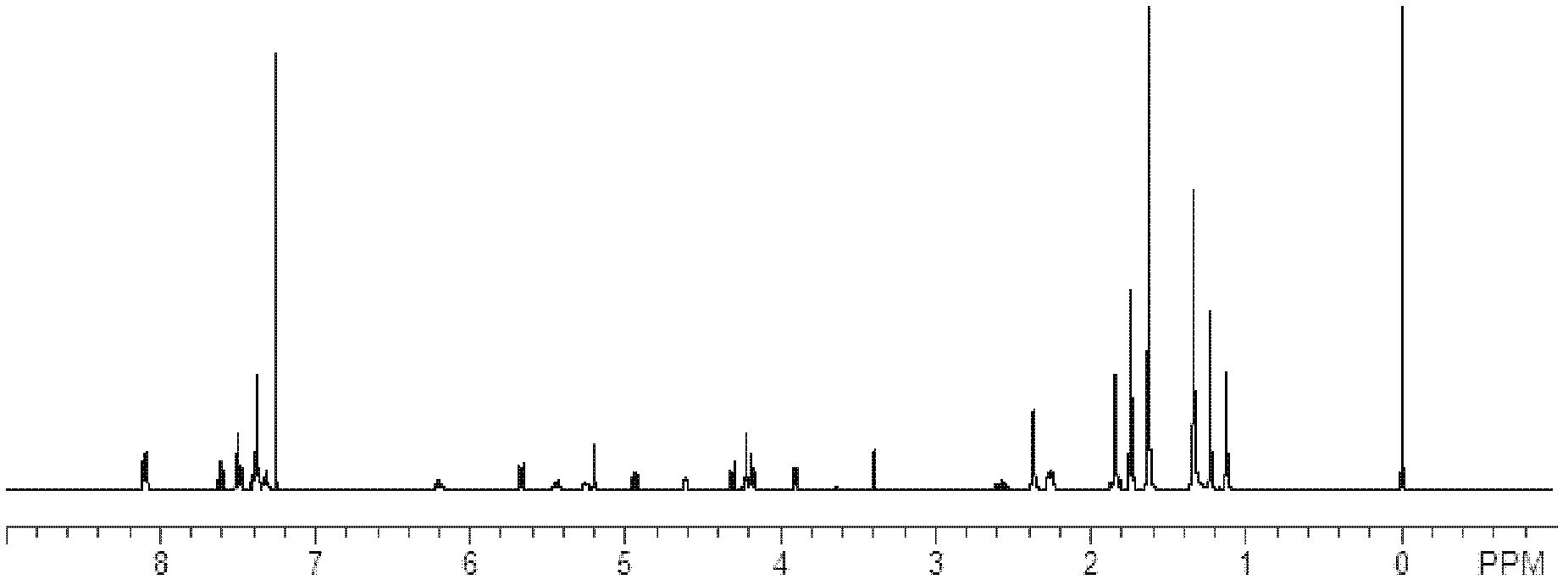
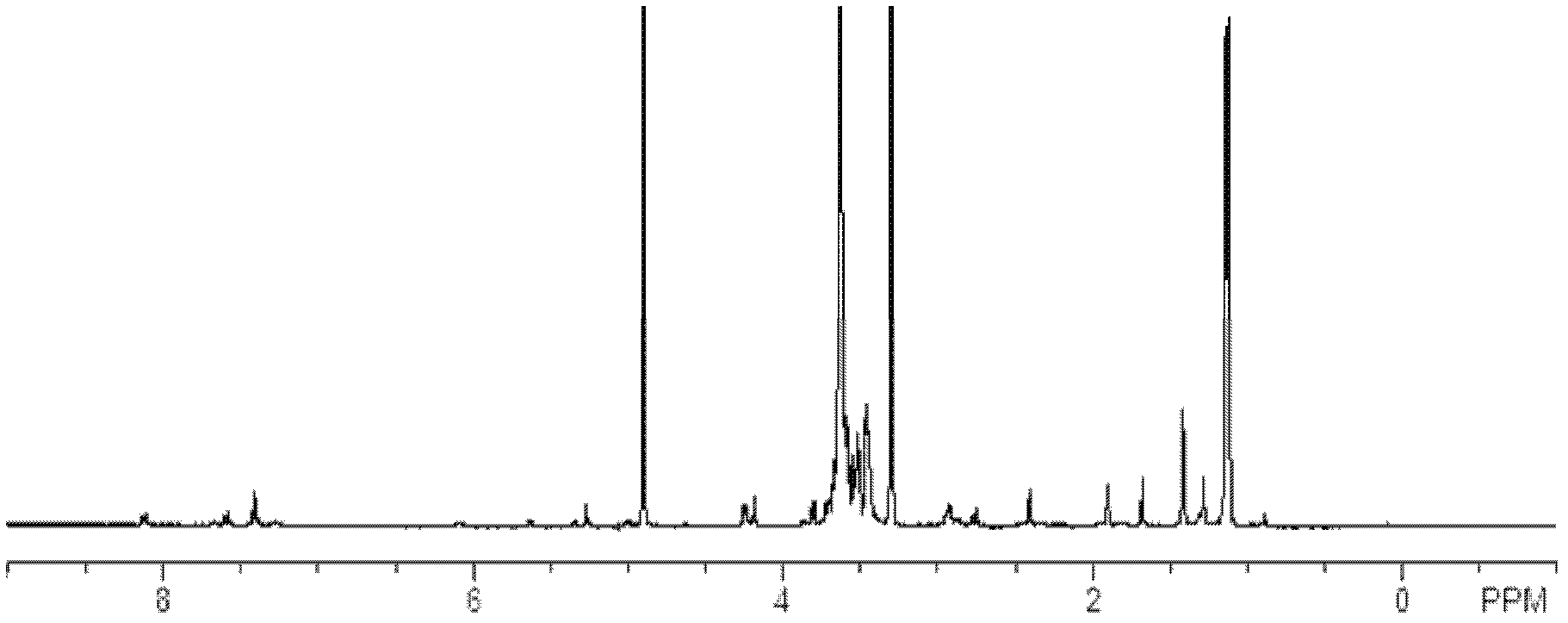
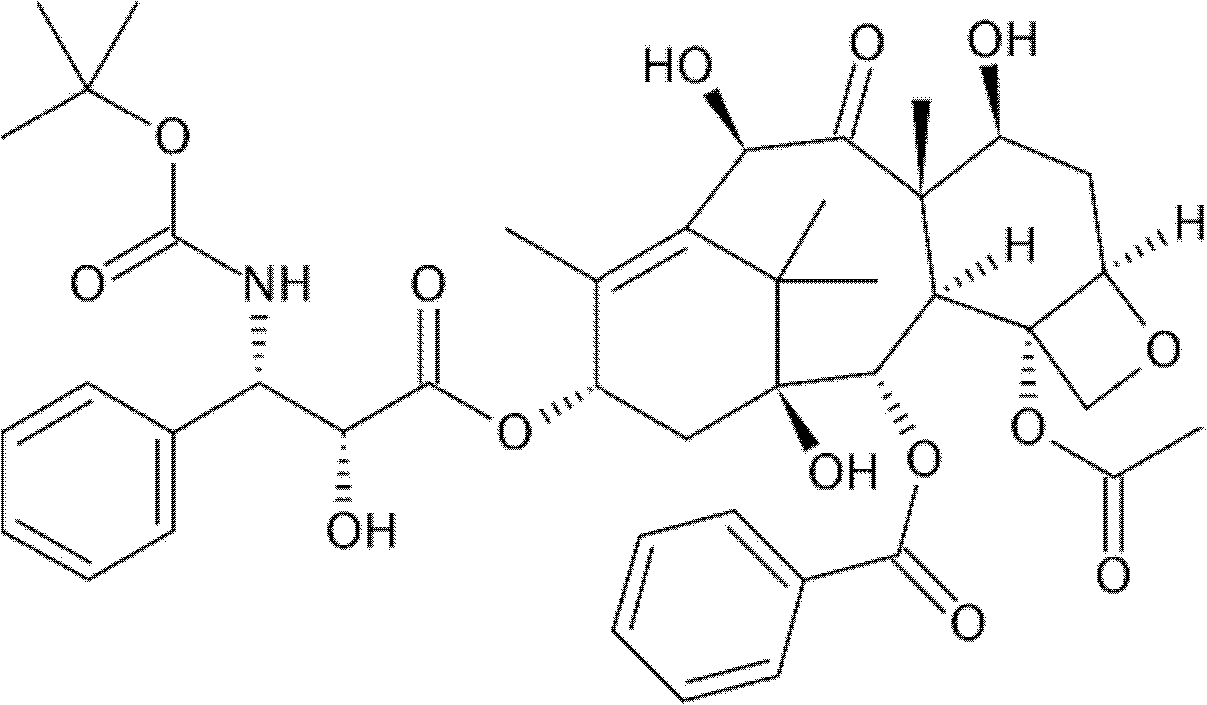
Docetaxelpolyoxyethelene-polyoxypropylene-polyoxyethelene compound capable of self-assembling into micelle
A technology of polyoxyethylene and docetaxel, which is applied in the direction of drug combination, medical preparation of non-active ingredients, drug delivery, etc., can solve the problems of reduced sensitivity, tumor drug resistance, rapid release, etc., and achieve simple compound composition , Avoid allergic reactions, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Take 50mg of thionyl chloride and 50mg of 3-3’-dithiodipropionic acid and mix them in a water bath at 30℃ for 12 hours. Add 10mg of docetaxel and continue the reaction for 6 hours. Add 0.5ml of triethylamine and 30mg of P85 (purchased from BASF) in a water bath at 25°C for 48 hours. After vacuum drying, dissolve the sample in absolute ethanol, add water for injection ultrasound, and place it in a 3500 Dalton dialysis bag for dialysis to obtain a micelle of compound A .
[0042] Using the different polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymers shown in Table 1, micelles of compounds B, C, F, G, H, J and K can be prepared in the same manner as above .
Embodiment 2
[0044] Take 10mg of docetaxel, 0.5ml of succinic anhydride and 30mg of polyoxyethylene-polyoxypropylene-polyoxyethylene polymer in a water bath at 25°C for 48 hours. After vacuum drying, dissolve the sample in absolute ethanol and add water for injection ultrasound. , Placed in a 3500 Dalton dialysis bag and dialyzed to obtain compound D micelles. Replace different polyoxyethylene-polyoxypropylene-polyoxyethylene polymers according to the table to obtain micelles of compound E and I.
[0045] Table 1 Various docetaxel polyoxyethylene-polyoxypropylene-polyoxyethylene compounds
[0046]
[0047] The particle size was measured with a Malvern particle size analyzer, model ZEW3690. Identify the formation of polyoxyethylene-polyoxypropylene-polyoxyethylene compound of docetaxel by high performance liquid chromatography: chromatographic column: BEH C18 column, mobile phase A is acetonitrile, mobile phase B is water, linear gradient elution , From the beginning to 3 minutes 50% mobile ph...
experiment example
[0060] The beneficial effects of the present invention are described by measuring the half inhibition rate and growth inhibition rate of docetaxel on the drug-resistant human breast cancer cell line MCF-7 / ADM.
[0061] Docetaxel reference substance: Docetaxel injection containing 20mg of docetaxel (trade name Taxotere, produced by French Aventis) is used to solubilize docetaxel with Tween 80 Used for injection.
[0062] Docetaxel micellar reference substance: take 20mg of docetaxel, add 5ml of dichloromethane to dissolve, take polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymer ( F68) 50mg, add 8ml of absolute ethanol to dissolve, mix the two solutions at 50℃, sonicate for 10 minutes, use a rotary evaporator to remove the organic solvent, and dissolve the residue with 5ml of water for injection at 37℃, namely docetaxel Micelles.
[0063] Docetaxel nanoparticle reference substance: take 20mg of docetaxel, add 5ml of absolute ethanol to dissolve, add 200mg of phospholipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com