Method for preparing recombinant human nerve growth factor by using Escherichia coli expression system
A technology of growth factor and Escherichia coli, which is applied in the field of preparation of recombinant human nerve growth factor, can solve problems such as inconsistency, small prokaryotic cells, and difficulty in achieving renaturation effects, and achieve simple and easy preparation methods, high purification efficiency, and easy implementation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Obtaining genetically engineered bacteria expressing the modified human nerve growth factor β subunit
[0051] 1. Referring to the preferred codon table of prokaryotes, without changing the amino acid sequence, the base sequence of the leader peptide and mature peptide of the natural human nerve growth factor β subunit was modified, and enterokinase was designed between the two sequences Cutting site, the sequence was synthesized by chemical synthesis (the base sequence is shown in SEQ ID NO: 3, and the expressed amino acid sequence is shown in SEQ ID NO: 4)
[0052] The codons formed by the bases in the sequence are all high-frequency codons of the E. coli expression system, which can maximize the expression of the target gene without changing the amino acid sequence. The 5' end GGATCC and the 3' end CTCGAG in the sequence are BamHI and XhoI restriction endonuclease sites respectively, which can facilitate the insertion of the target gene into the pET28a(+) ...
Embodiment 2
[0059] Example 2: Screening of Induction Conditions for rhβNGF Genetically Engineered Bacteria
[0060] Inoculate 0.2% recombinant engineered bacteria frozen in glycerol tubes into fresh LB-resistant medium (containing Kana 50 μg / ml), culture overnight at 37°C with shaking at 220 rpm, and activate the engineered bacteria.
[0061] 1. Effect of IPTG concentration on protein expression
[0062] Inoculate the activated engineered bacteria into 2ml of LB-resistant medium (containing Kana 50μg / ml), shake culture at 37°C and 220rpm until A550=0.6, then add IPTG to make the final concentrations 0.1 mmol / L, 0.5 mmol / L, respectively / L, 1.0 mmol / L, 1.5 mmol / L, 2.0 mmol / L, 3.0 mmol / L, 4.0 mmol / L, 5.0 mmol / L, after 3 hours of induction culture, centrifuge to collect the precipitate, and use 0.5ml, 20mmol / L Tris -HCl (pH8.0) resuspended cells, repeated freezing and thawing at -80°C for three times, centrifuged to take the precipitate, resuspended with 0.1ml protein electrophoresis sample...
Embodiment 3
[0070] Example 3: Induced expression of rhβNGF genetically engineered bacteria and isolation and purification of inclusion bodies
[0071] 1. Pick a monoclonal genetically engineered colony and inoculate it in 50mL LB liquid medium, shake at 220rpm at 37°C for 14h;
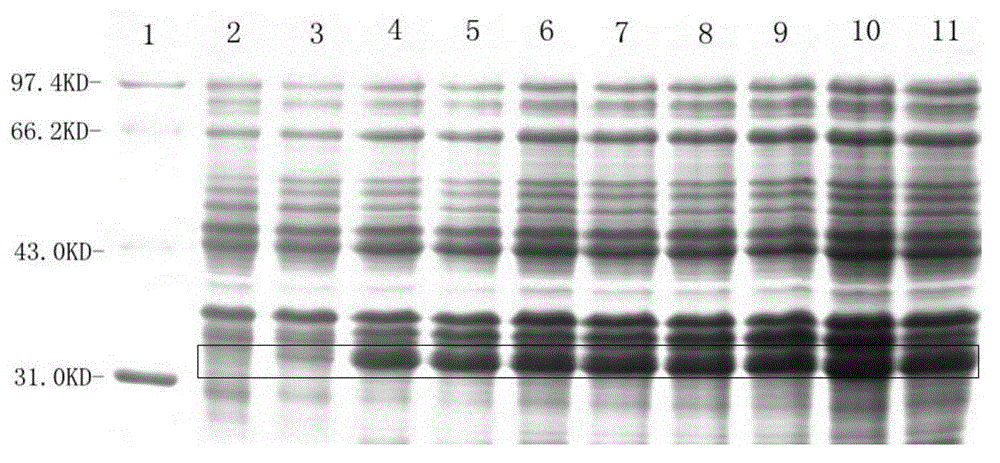
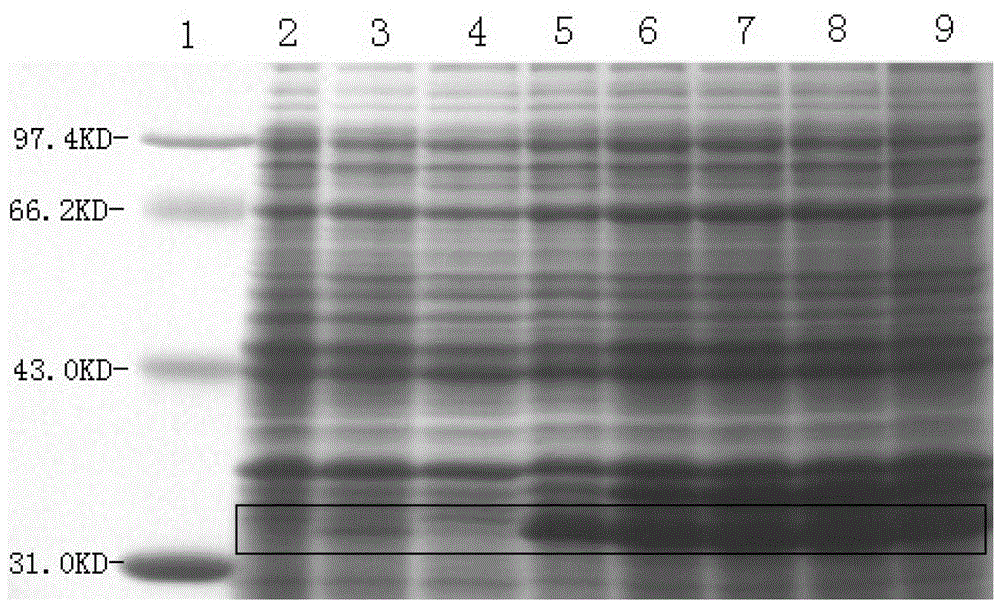
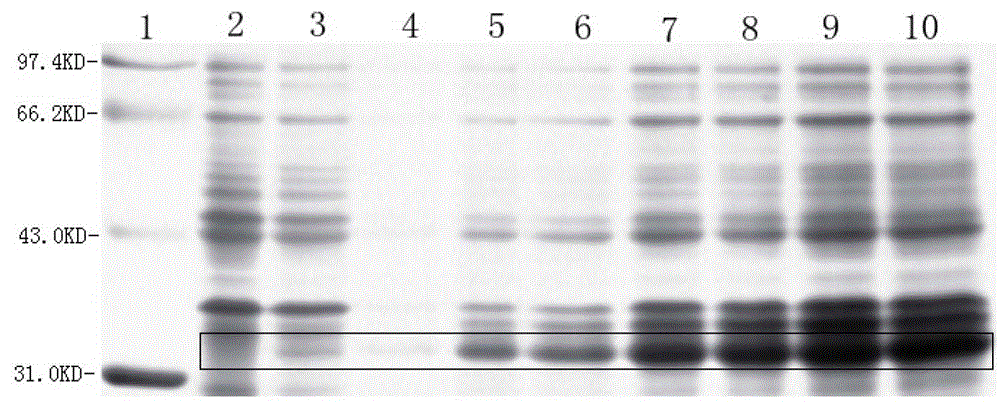
[0072] 2. Cultivate the above bacteria in 3L liquid LB medium at 37°C and 220rpm shaking until A550=0.6 (about 3h), according to the results of the induction conditions (such as figure 1 , figure 2 , image 3 ), adding IPTG with a final concentration of 1mM, maintaining the pH value of the medium at 7.0, and inducing at 37°C for 3.5 hours;
[0073] 3. Centrifuge (4°C, 8000rpm, 10min), collect the cells, add 20mM Tris-HCl (pH=8.0) lysis buffer to resuspend at a ratio of 20ml per gram of cell wet weight, and freeze at -20°C;
[0074] 4. Dissolve the bacterial suspension at 37°C, and perform ultrasonic lysis, with a power of 400W, ultrasonic for 15 seconds, with an interval of 15 seconds, for 30 minutes.
[0075...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com