Halamine antibacterial agent and synthetic method and application thereof
A synthesis method and technology for an antibacterial agent are applied in the synthesis and antibacterial fields of halamine compounds, which can solve the problems of increasing finishing costs and wastewater treatment costs, poor water solubility of synthetic products, and large reaction energy consumption, and reduce the cost of antibacterial finishing, The effect of high product yield and low reaction energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
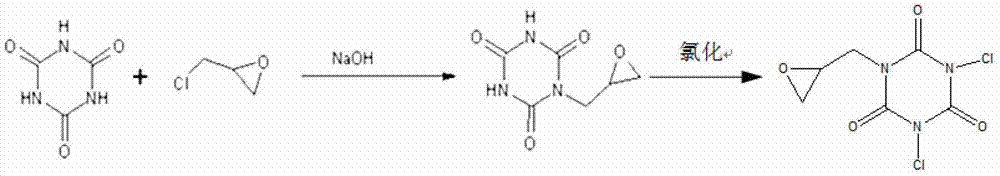
[0048] Synthesis of Halamine Antibacterial Agent 1,3-Dichloro-5-epoxypropyl-S-triazinetrione
[0049] Weigh 6.45g (0.05mol) of cyanuric acid into a 250mL flat-bottomed flask, add 100mL of deionized water, stir evenly with a glass rod, add 4g of sodium hydroxide, continue stirring until the cyanuric acid is completely dissolved, slowly add 4.63g (0.05 mol) epichlorohydrin, fully stirred and reacted at room temperature (25°C) for 6 hours, and adjusted the pH value of the reaction solution to 6.5 with dilute hydrochloric acid; removed the water in the reaction solution with a rotary evaporator, and dissolved the obtained solid in dimethyl Dimethyl formamide (DMF), filtered according to the conventional method to remove the synthetic by-product sodium chloride, and then remove the DMF in the filtrate by a rotary evaporator to obtain a water-soluble colorless oily haloamine antibacterial agent precursor 1-epoxy Propyl-S-triazinetrione; Finally, add the precursor to a sodium hypochl...
Embodiment 2
[0051] Synthesis of Halamine Antibacterial Agent 1-Chloro-3,5-Diepoxypropyl-S-Triazinetrione
[0052] Weigh 6.45g (0.05mol) of cyanuric acid into a 250mL flat-bottomed flask, add 100mL of deionized water, stir evenly with a glass rod, add 5.6g of potassium hydroxide, continue stirring until the cyanuric acid is completely dissolved, slowly add 9.25g ( 0.1mol) of epichlorohydrin, fully stirred and reacted at 5°C for 12 hours, and adjusted the pH value of the reaction solution to 7.0 with dilute sulfuric acid; removed the water in the reaction solution with a rotary evaporator, and dissolved the obtained solid in ethanol, according to Filtrate by conventional method to remove the synthetic by-product potassium chloride, then remove the ethanol in the filtrate by rotary evaporator to obtain the water-soluble colorless oily haloamine antibacterial agent precursor 1,3-diepoxypropyl-S-tri Azitrione; finally add the precursor to a sodium hypochlorite solution with a concentration of ...
Embodiment 3
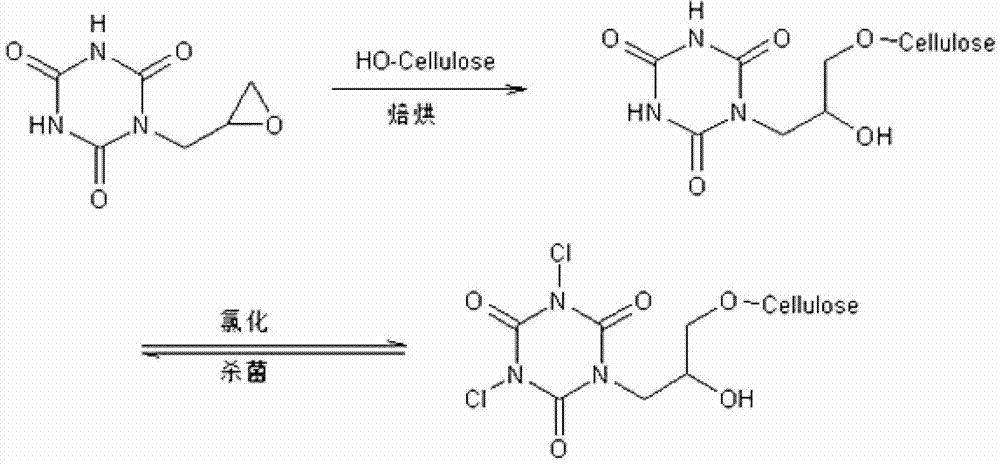
[0054] Synthesis of Halamine Antibacterial Agent 1-Chloro-3-Hydroxymethyl-5-Epoxypropyl-S-Triazinetrione
[0055] Weigh 15.9g (0.1mol) of 1-hydroxymethyl-S-triazinetrione (that is, a compound in which one of the hydrogen atoms of cyanuric acid is replaced by a hydroxymethyl group) into a 250mL flat-bottomed flask, add 100mL of deionized water, and use After the glass rod is stirred evenly, add 30g of sodium hydroxide, continue to stir until 1-hydroxymethyl-S-triazinetrione is completely dissolved, slowly add 9.25g (0.1mol) of epichlorohydrin, and fully stir at 50°C for 9h , and adjust the pH value of the reaction solution to 7.5 with dilute sulfuric acid; remove the water in the reaction solution with a rotary evaporator, and dissolve the obtained solid in dimethylacetamide (DMAC), and filter according to the conventional method to remove the synthetic by-product Product sodium chloride, removes ethanol in the filtrate by rotary evaporator again, obtains water-soluble colorles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com