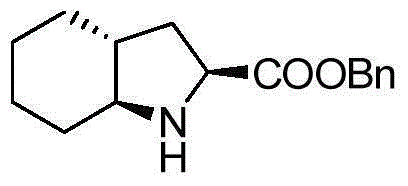
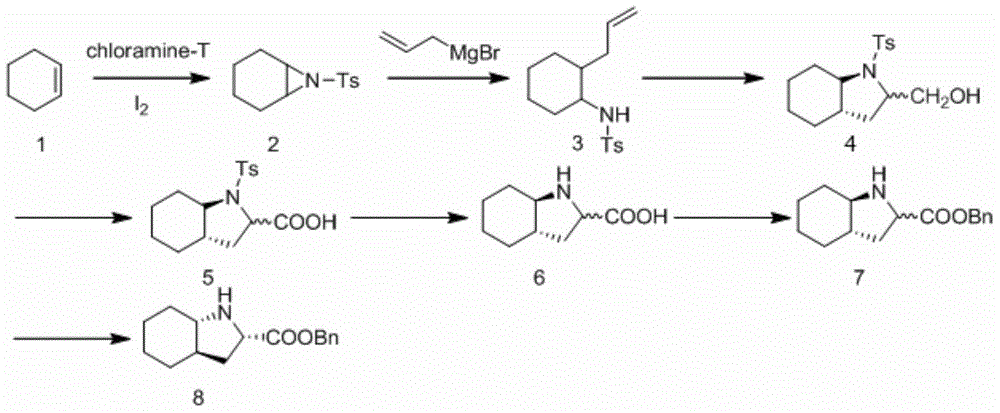
A kind of preparation method of trandolapril intermediate
A technology for trandolapril and intermediates, which is applied in the new field of preparation of key intermediates, can solve the problems of long process route, long reaction time, expensive catalyst, etc., and achieve reasonable preparation process, short preparation method route and good three-dimensional selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038](1) Synthesis of cyclohexaneziridine (hereinafter referred to as compound 2)
[0039] Chloramine T (500g, 1.775mol), cyclohexene (164g, 2.0mol), iodine (28.2g, 0.11mol) and benzyltrimethylammonium bromide (20.4g, 0.088mol) were added to 1000mL tetrahydrofuran and 500mL water In a mixed solvent, react at room temperature for 24 hours, distill off tetrahydrofuran, extract the aqueous phase with ethyl acetate (500mL×3), combine the organic phases, dry with anhydrous sodium sulfate, filter with suction, remove the solvent by rotary evaporation, and reconstitute the product with ethanol Crystallization gave white crystals (260g, 58%).
[0040] (2) Synthesis of trans-N-p-methylbenzenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3)
[0041] Dissolve cyclohexaneziridine (25.1g, 0.1mol) and copper bromide (2.23g, 0.01mol) in 200mL ether, and allylmagnesium bromide (29g, 0.2mol) at -40°C, Add dropwise slowly and stir overnight. The reaction was q...
Embodiment 2
[0055] (1) Synthesis of cyclohexaneziridine (hereinafter referred to as compound 2)
[0056] Chloramine T (500g, 1.775mol), cyclohexene (164g, 2.0mol), iodine (28.2g) and benzyltrimethylammonium chloride (16.5g, 0.088mol) were added to a mixed solvent of 1000mL tetrahydrofuran and 500mL water In the reaction at room temperature for 24h. The tetrahydrofuran was evaporated, the aqueous phase was extracted with ethyl acetate (500mL×3), the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, the solvent was removed by rotary evaporation, and the product was recrystallized with ethanol to obtain white crystals (270g, 60%).
[0057] (2) Synthesis of trans-N-p-methylbenzenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3)
[0058] Dissolve cyclohexaneziridine (25.1g, 0.1mol) and copper bromide (2.23g, 0.01mol) in 200mL ether, and allylmagnesium bromide (58g, 0.4mol) at -50°C, Add dropwise slowly and stir overnight...
Embodiment 3
[0072] (1) Synthesis of cyclohexaneziridine (hereinafter referred to as compound 2)
[0073] Chloramine T (500g, 1.775mol), cyclohexene (164g, 2.0mol), iodine (28.2g) and benzyltrimethylammonium chloride (16.5g, 0.088mol) were added to a mixed solvent of 1000mL acetonitrile and 500mL water In the reaction at room temperature for 24h. Acetonitrile was evaporated, the aqueous phase was extracted with ethyl acetate (500mL×3), the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, and the solvent was removed by rotary evaporation, and the product was recrystallized with ethanol to obtain white crystals (275g, 61%).
[0074] (2) Synthesis of trans-N-p-methylbenzenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3)
[0075] Dissolve cyclohexaneziridine (25.1g, 0.1mol) and copper bromide (2.23g, 0.01mol) in 200mL tetrahydrofuran, and place allylmagnesium bromide (58g, 0.4mol) at -50°C, Add dropwise slowly and stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com