Purification method of 4N-purity nitric oxide gas
A nitric oxide and purification method technology, which is applied in the fields of nitrous oxide capture, chemical instruments and methods, and greenhouse gas capture, can solve the problems of high impurity content, consumption of nitric oxide, hidden dangers, etc., and achieve simple operation process, Effect of inhibiting oxidation reaction and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
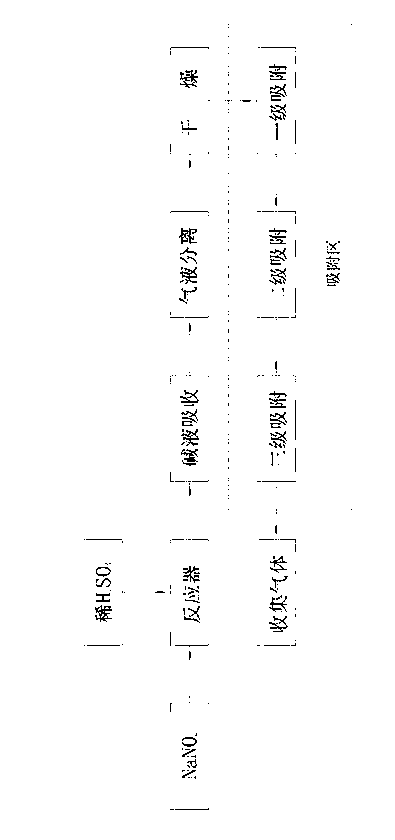
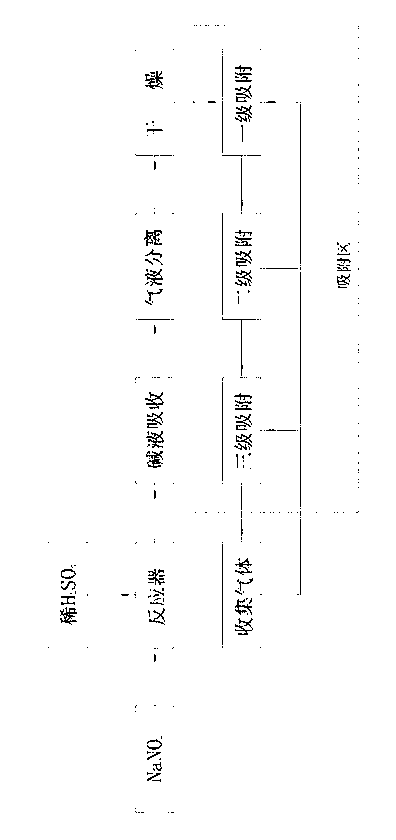
[0016] Same as the prior art, react sodium nitrite and dilute sulfuric acid in the reaction tank, the remaining reaction solution and the generated sodium sulfate and sodium nitrate solution and a small amount of CO 2 , SO 2 and a small fraction of NO 2 Separation and removal from the bottom of the reactor by alkaline absorption and water washing, most of the NO 2 , N 2 O, water vapor, etc. flow out from the top of the reactor in gaseous form along with NO; water vapor is absorbed and removed in the dryer where the gas flow passes through the presence of anhydrous calcium chloride. The present invention differs from the prior art in that polyethylene glycol dimethacrylate is used as the adsorbent to absorb NO 2 , N 2 O impurity. Process such as figure 1 Shown: Including placing dilute sulfuric acid and sodium nitrite in a reactor for reaction, lye absorption, gas-liquid separation, and drying to obtain gas. Under the condition of 1.0MPa, it passes through the polyethyle...
Embodiment 2
[0020] The basic process is the same as in Example 1, except that the pressure in the polyethylene glycol dimethacrylate adsorption zone is 0.6 MPa to 0.8 MPa, and the adsorption zone is divided into three zones, namely primary adsorption, secondary adsorption, and tertiary adsorption . The pressure of the first-stage adsorber is best adjusted at 0.8MPa, and the polyethylene glycol-methacrylate copolymer adsorbent takes an average of 500m 2 / g The specific surface area exists in the form of a coarse-pore network, which is fixed at the bottom of the adsorbent through a quartz layer; the pressure of the secondary adsorber is best adjusted at 0.7MPa, and the polyethylene glycol-methacrylate copolymer adsorbent takes an average of 450m 2 / g The specific surface area exists in the form of a coarse-pore network, which is fixed at the bottom of the adsorbent through the quartz layer; the pressure of the third-stage adsorber is best adjusted at 0.6MPa, and the polyethylene glycol-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com