Preparation of VO2 nanosheet material and applications thereof
A VO2 and nanosheet technology, applied in the field of VO2 nanosheet materials and their preparation, to achieve the effects of uniform crystal phase, uniform morphology, high activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Material A (X-100-10)
[0025] Take 30g of ethylene glycol and 30g of water and mix and stir for 10min, then add 1g of V 2 o 5 Stir for 10min, add the mixed solution into a reaction kettle lined with polytetrafluoroethylene, and pass through N 2 Air for 1 min. The resulting mixed solution was sealed, put into an oven at 100° C., and subjected to hydrothermal reaction for 10 h. The obtained material was first centrifuged, poured out the supernatant, added 20ml of water, ultrasonicated for 10min, centrifuged again, poured out the supernatant, washed with water for 3 times, then added 20ml of ethanol and washed with ethanol for 3 times, and then washed in 80°C VO was obtained after drying under vacuum conditions 2 Nanosheet material X-100-10.
Embodiment 2
[0026] Example 2 Material B
[0027] The preparation method of material B-I is the same as that of material A, except that the temperature and time of hydrothermal reaction are different. The specific temperature and time are shown in Table 1, and the obtained materials are listed in Table 1.
[0028] Table 1 The temperature and time of the hydrothermal reaction adopted in the preparation of material B-I
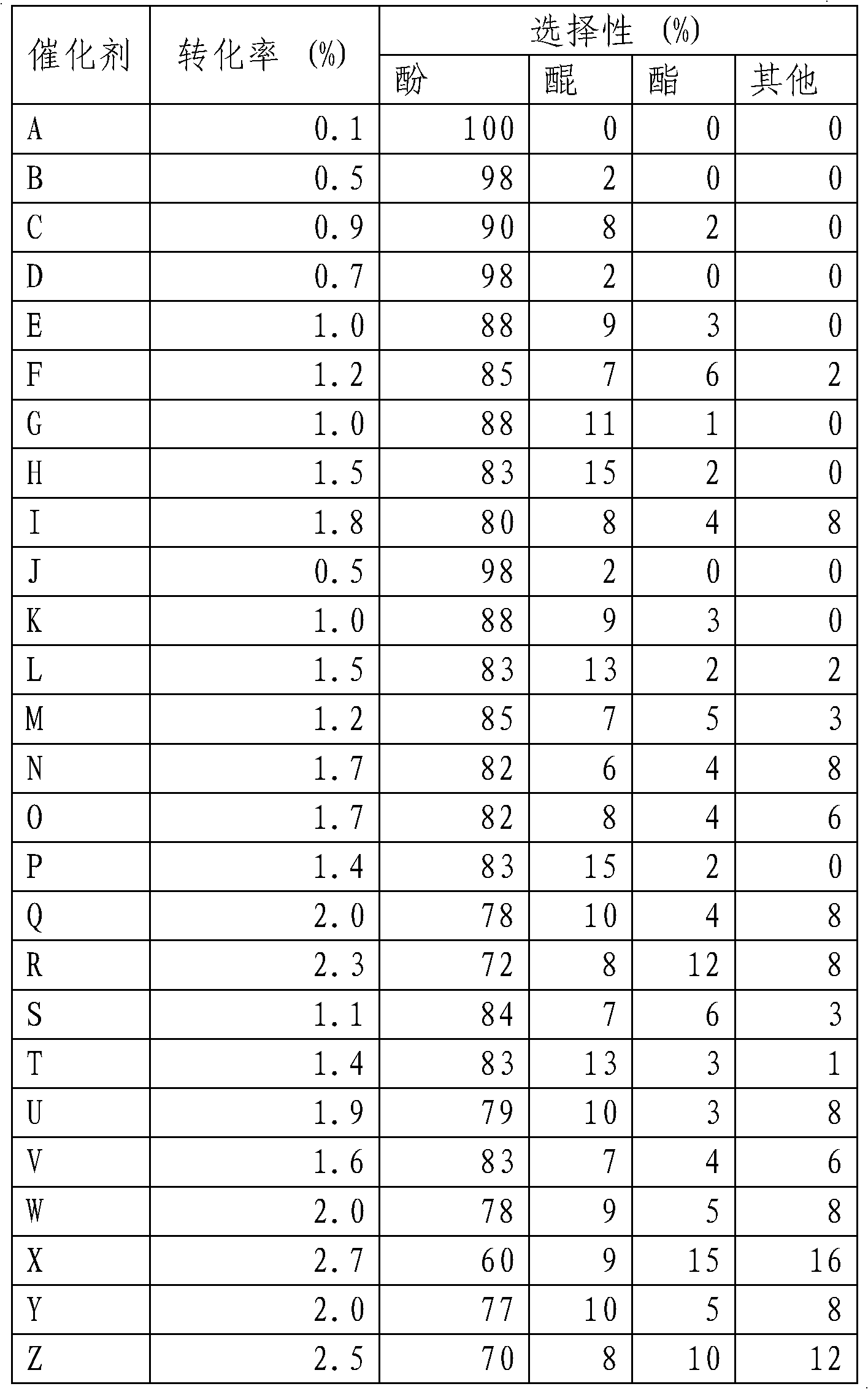
[0029] material code Water temperature (℃) Water heating time (h) material name B 100 20 X-100-20 C 100 40 X-100-40 D 140 10 X-140-10 E 140 20 X-140-20 F 140 40 X-140-40 G 180 10 X-180-10 H 180 20 X-180-20 I 180 40 X-180-40
Embodiment 3
[0030] The preparation of embodiment 3 material J (Y-100-10)
[0031] Take 30g xylitol, 30g water and mix and stir for 10min, then add 1g NH 4 VO 3 Stir for 10min, add the mixed solution into a reaction kettle lined with polytetrafluoroethylene, and pass through N 2 Air for 1 min. The resulting mixed solution was sealed, put into an oven at 100° C., and subjected to hydrothermal reaction for 10 h. The obtained material was first centrifuged, poured out the supernatant, added 20ml of water, ultrasonicated for 10 minutes, centrifuged again, poured out the supernatant, washed repeatedly for 3 times, then added 20ml of ethanol for 3 times, and then vacuumed at 80 degrees VO after drying 2 Nanosheet material Y-100-10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com