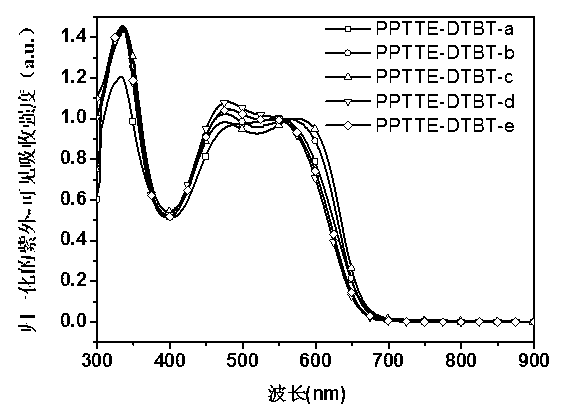
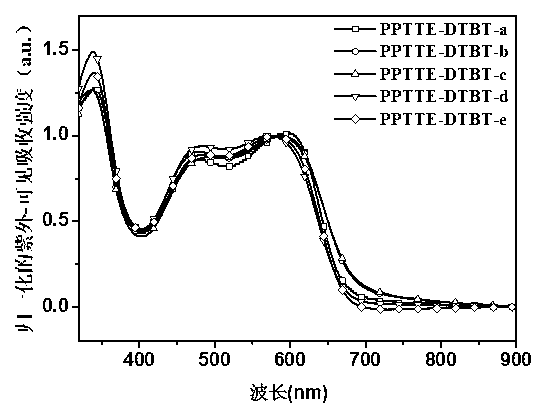
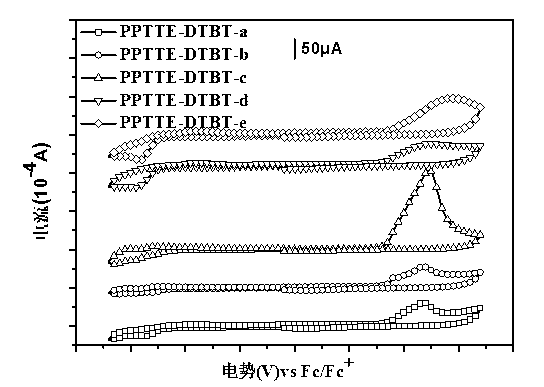
Perylene tetracarboxylic carboxylic ester group polymer acceptor materials and application thereof to solar battery
A technology of perylene tetracarboxylate and acceptor materials, applied in the field of organic optoelectronics, which can solve the problems of limited influence and adjustment ability, low battery open circuit voltage, weak absorption of fullerene derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Poly-3,4,9,10-perylenetetracarboxylate-tetrakis(2-hexyldecyl)-alt-4,7-bis(thiophen-2-)yl[c]2,1,3-benzothiabis Preparation of azole (abbreviated as PPTTE-DTBT-a)
[0027] The synthetic route is as follows:
[0028]
[0029] (1) Preparation of 3,4,9,10-perylenetetracarboxylate-tetra(2-hexyldecyl) ester
[0030] Add 3.92g of PTCDA (10mmol) to a 250mL two-necked flask, then add 100mL of acetonitrile solution, 14.52g of 2-hexyldecanol (60mmol), 18.32g of 1-bromo-2-hexyldecane (60mmol) and 2.43g of DBU (80mmol), heated to reflux, monitored by TLC, and then cooled to room temperature. Washed with water, extracted with petroleum ether, and concentrated the organic phase. Distilled under reduced pressure to remove 2-hexyldecanol, and the crude product was subjected to silica gel column chromatography to obtain 9.93 g of pure compound (1), with a yield of 75%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ[ppm]: 8.33(d, J=8.1Hz, 4H), 8.04(d, J=7.9Hz, 4H), 4.23(d, J=6.0Hz, 8H), 1.82...
Embodiment 2
[0042] Poly-3,4,9,10-perylenetetracarboxylate-tetrakis(2-ethylhexyl)-alt-4,7-di(thiophen-2-)yl[c]2,1,3-benzothiabis Preparation of azole (abbreviated as PPTTE-DTBT-b)
[0043] The synthetic route is as follows:
[0044]
[0045] The synthetic method of compound (4) in the synthetic route is the same as that of compound (1) in Example 1, with 2-ethylhexanol and 1-bromo-2-ethylhexane instead of 2-hexyldecanol and 1-bromo-2 - Hexyldecane. The synthesis of compound (5) is the same as that of compound (2) in Example 1.
[0046] (1) Poly-3,4,9,10-perylenetetracarboxylate-tetrakis(2-ethylhexyl)-alt-4,7-di(thiophen-2-)yl[c]2,1,3-benzene Preparation of thiadiazole
[0047] Under nitrogen protection, 0.517g 1,7-dibromo-3,4,9,10-perylenetetracarboxylate-tetrakis(2-ethylhexyl)ester (0.5mmol), 0.313g 4,7-bis( 2-trimethyltinyl-thiophen-5-)yl[c]2,1,3-benzothiadiazole (0.5mmol), 14.0mgPd 2 (dba) 3 and 27.4mg P(o-Tol) 3 Add it into a 50mL single-necked bottle, then add 20mL of anhyd...
Embodiment 3
[0050] Poly-3,4,9,10-perylenetetracarboxylate-tetrakis(2-octyl)-alt-4,7-bis(thiophen-2-)yl[c]2,1,3-benzothiadiazole Preparation (abbreviated as PPTTE-DTBT-c)
[0051] The synthetic route is as follows:
[0052]
[0053] The synthesis method of compound (6) in the synthetic route is the same as that of compound (1) in Example 1, with 1-octanol and 1-bromooctane instead of 2-hexyldecanol and 1-bromo-2-hexyldecane. The synthesis method of compound (7) is the same as that of compound (2) in Example 1. The synthetic method of polymer is the same as embodiment 2, replaces 1,7-dibromo-3,4 with 1,7-dibromo-3,4,9,10-perylenetetracarboxylic acid-tetra(2-octyl) ester, 9,10-Perylenetetracarboxylate-tetra(2-ethylhexyl) ester.
[0054] The polymer structure is characterized as follows: GPC: Mn=105.59K, Mw=311.187K, PDI=2.94, n=90. Elemental analysis C 70 h 80 N 2 o 8 S 3 Calculated for: C, 71.67; H, 6.82; N, 2.38; S, 8.19; Found: C, 70.16; H, 6.76; N, 2.35;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com