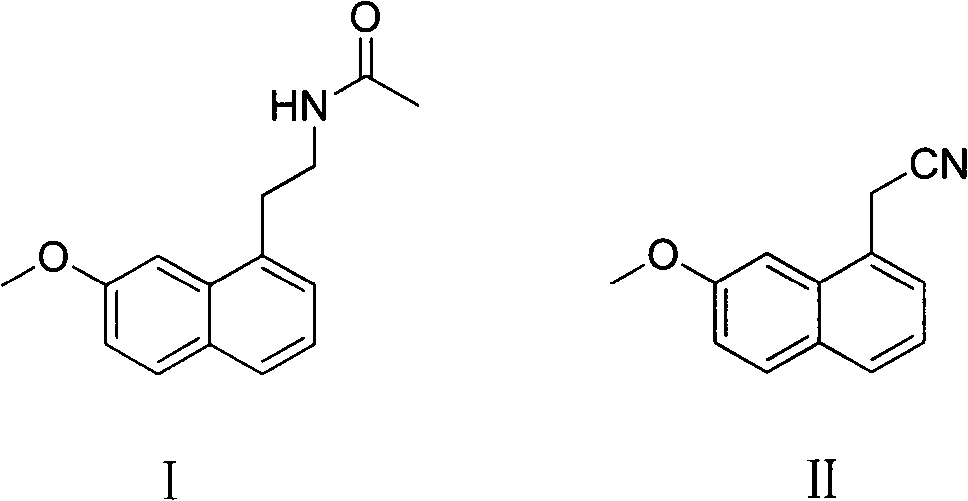
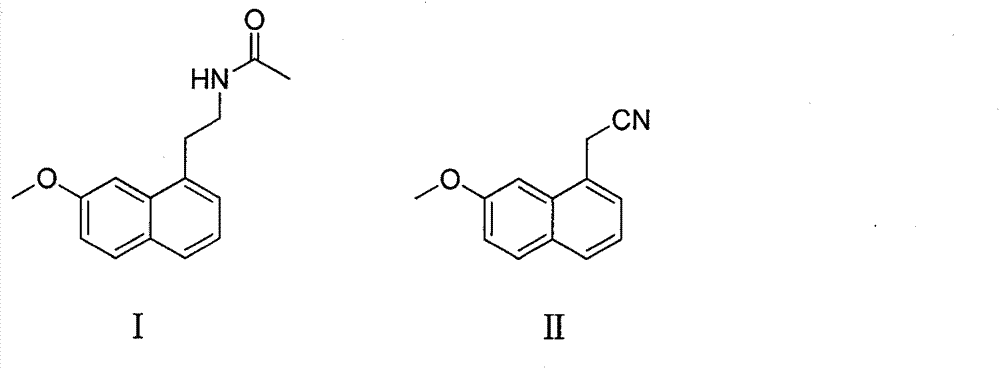
A kind of production method of hydrogenation preparation agomelatine
A production method and hydrogen technology, which are used in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve problems such as difficult to achieve, and achieve the effects of improving reaction yield, reducing pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of agomelatine (compound I) by using acetyl chloride as acylating agent and triethylamine as acid-binding agent
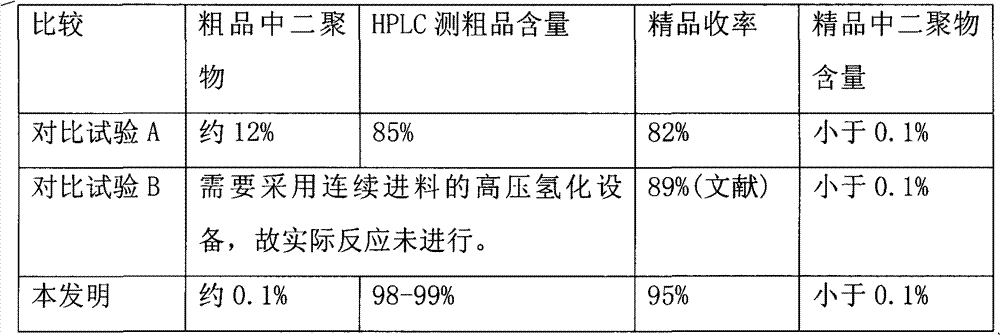
[0032] In a dry 100L autoclave, add (7-methoxyl-1-naphthyl) acetonitrile 9.85kg (50mol), anhydrous tetrahydrofuran 39.4L, Raney-Ni 1.2kg, acetyl chloride 4.32Kg (55mol), triethylamine 11.1Kg (110mol), stir evenly, vacuumize and replace with nitrogen, vacuumize and replace with hydrogen (gas supplied by industrial steel cylinders, purity> 99%), control the hydrogen pressure at 1-1.5Mpa, control the temperature at 45°C, and react for 2 hours. After the reaction is complete, suck out the reaction solution from the autoclave, evaporate to dryness under reduced pressure, dissolve in ethyl acetate, wash with saturated sodium bicarbonate water until neutral, leave to separate layers, and evaporate the organic phase to dryness under reduced pressure to obtain the compound I, weighing 11.5kg, the purity is 99% (HPLC), and the yield of crude product is 95%...
Embodiment 2
[0034] Use acetic anhydride as an acylating agent and pyridine as an acid-binding agent to prepare agomelatine (compound I). In a dry 100L autoclave, add (7-methoxy-1-naphthyl) acetonitrile 9.85kg (50mol) , anhydrous tetrahydrofuran 39.4L, Raney-Ni 1kg, acetic anhydride 5.6Kg (55mol), pyridine 8.7Kg (110mol), stir evenly, vacuumize and replace with nitrogen, vacuumize and replace with hydrogen (gas supplied by industrial steel cylinders, purity > 99 %), the hydrogen pressure is controlled at 1-1.2Mpa, the temperature is controlled at 40°C, and the reaction is carried out for 1.5 hours. After the reaction was completed, the reaction solution was sucked out from the autoclave, evaporated to dryness under reduced pressure, the residue was dissolved in ethyl acetate, washed with saturated aqueous sodium bicarbonate until neutral, left to separate layers, and the organic phase was evaporated to dryness under reduced pressure to obtain the compound 1, crude product 11.9kg, purity 99...
Embodiment 3
[0036] Preparation of agomelatine (compound I) with toluene as reaction solvent, acetic anhydride as acylating agent and triethylamine as acid-binding agent
[0037] In a dry 100L autoclave, add (7-methoxyl-1-naphthyl) acetonitrile 9.85kg (50mol), anhydrous tetrahydrofuran 39.4L, Raney-Ni 1kg, acetic anhydride 5.6Kg (55mol), triethylamine 11.1 Kg (110mol), after stirring evenly, vacuumize and replace with nitrogen, vacuumize and replace with hydrogen (gas supplied by industrial steel cylinders, purity > 99%), control the hydrogen pressure at 1-1.5Mpa, control the temperature at 60°C, and react for 3 hours. After the reaction was completed, the reaction liquid was sucked out from the autoclave, and after being evaporated to dryness under reduced pressure, the residue was dissolved in ethyl acetate, washed with saturated aqueous sodium bicarbonate until neutral, allowed to stand for layers, and the organic phase was evaporated to dryness under reduced pressure. Compound I was ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com