Compound omeprazole capsule and preparation method and detection method thereof
The technology of omeprazole and detection method is applied in the field of compound omeprazole capsules and preparation thereof, and can solve the problem that there is no good solution for controlling the relative humidity of medicine powder mixing uniformity and filling environment, and sodium bicarbonate has adsorption Moisture, not easy to mix evenly, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Embodiment 1: Each compound omeprazole capsule is made of 20mg omeprazole, 1100mg sodium bicarbonate, 30mg magnesium stearate and 10mg talcum powder. Weigh the sodium bicarbonate of prescription, pulverize it, and screen it through a 60-mesh sieve for subsequent use; weigh the omeprazole of prescription, mix it with sodium bicarbonate by equal increment method Finally, add magnesium stearate and talcum powder, and further mix thoroughly; the above mixture is tested and packed into No. 00 ordinary capsules; during the mixing and capsule filling process, the ambient humidity is controlled below 55%.
Embodiment 2
[0269] Embodiment 2: Each compound omeprazole capsule is made of 40mg omeprazole, 1100mg sodium bicarbonate, 10mg magnesium stearate and 10mg talcum powder. Weigh the sodium bicarbonate of prescription, pulverize it, and screen it through a 60-mesh sieve for subsequent use; weigh the omeprazole of prescription, mix it with sodium bicarbonate by equal increment method Finally, add magnesium stearate and talcum powder, and further mix thoroughly; the above mixture is tested and packed into No. 00 ordinary capsules; during the mixing and capsule filling process, the ambient humidity is controlled below 55%.
Embodiment 3
[0270] Embodiment 3: the complete detection method of compound recipe omeprazole capsule of the present invention is:
[0271] (1) Properties: This preparation is white or off-white powder.
[0272] (2) Check:
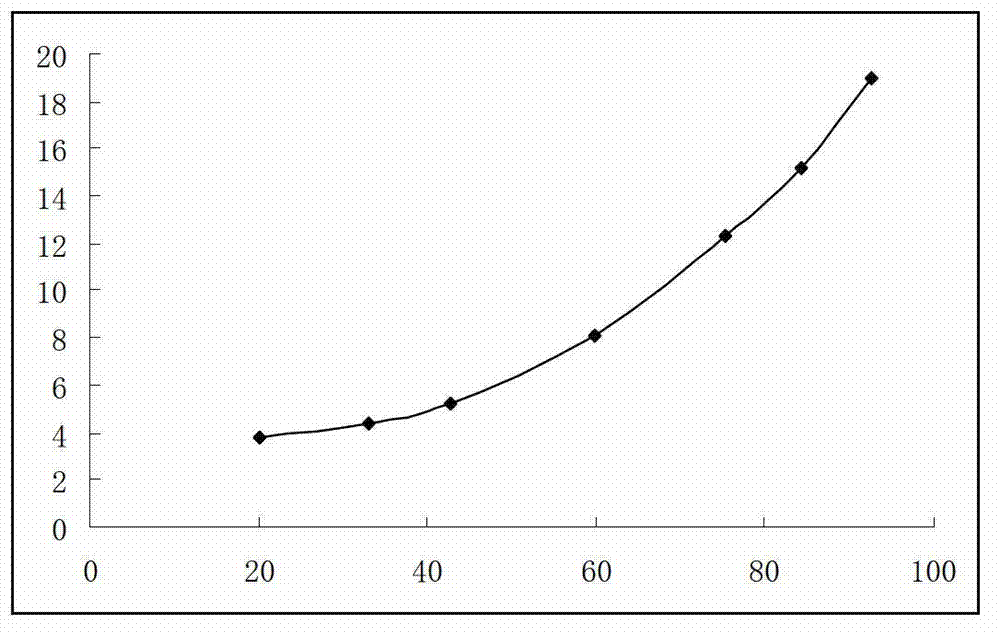
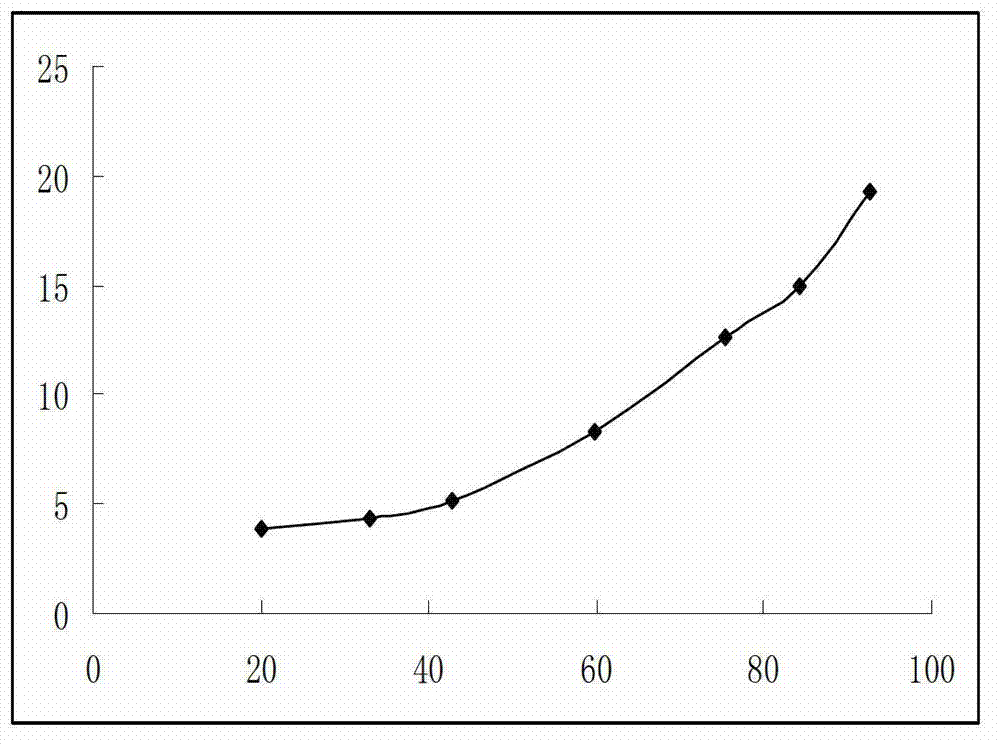
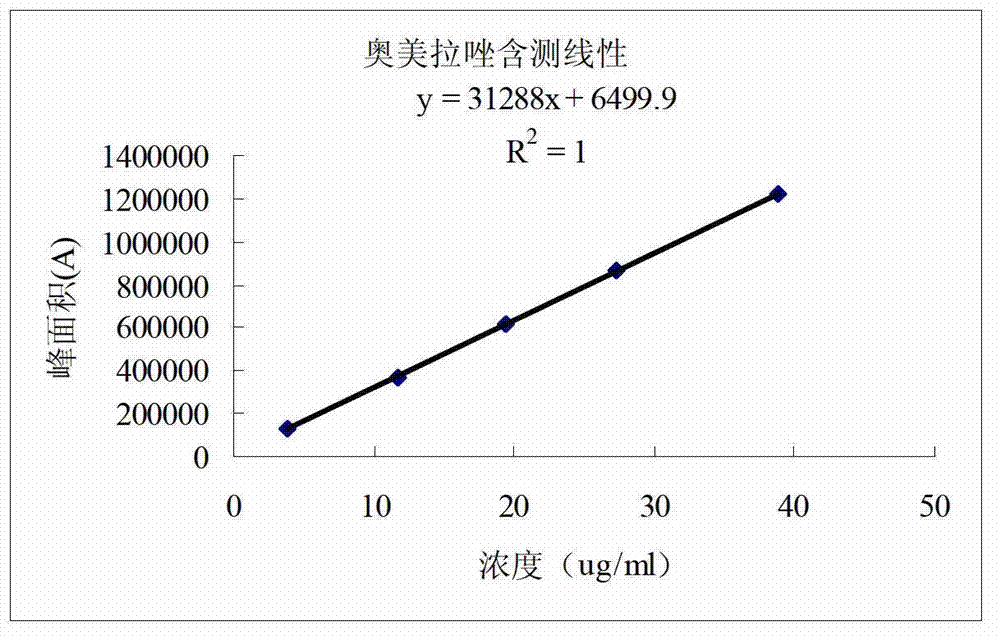
[0273](1) Dissolution: The instrument adopts the ZRS-8G drug dissolution apparatus; this preparation is the preferred basket method for capsules, and the paddle method is used for comparison at the same time; take this preparation, refer to the second method of Appendix X C of the 2010 edition of the Chinese Pharmacopoeia, Take 1000mL of water as the dissolution medium, the rotating speed is 100 revolutions per minute, operate according to the law, after 45 minutes, take 10mL of the solution, filter, accurately measure the subsequent filtrate and dilute with water to make a solution containing 10 μg of omeprazole per 1mL as a test Reference substance solution; refer to "Chinese Pharmacopoeia" 2010 edition two appendix Ⅳ A ultraviolet-visible spectrophotometry, measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com