Pyridoxal derivative for pegylation modification of N terminal of protein and preparation method and application thereof
A technology of polyethylene glycol and protein, applied in the field of chemical synthesis and modification of protein, can solve the problems of long reaction time and low yield, and achieve the goal of improving efficiency, improving transamination efficiency, and shortening reaction transamination time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
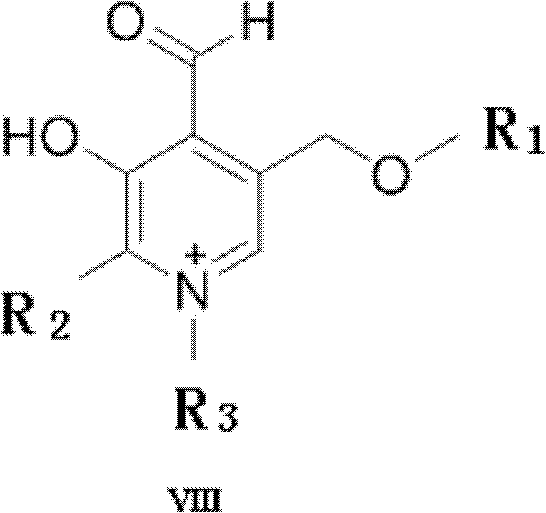
[0038] Preparation of 4-formyl-3-hydroxy-5-(methoxymethyl)-1,2-lutidine salt of formula VIII:
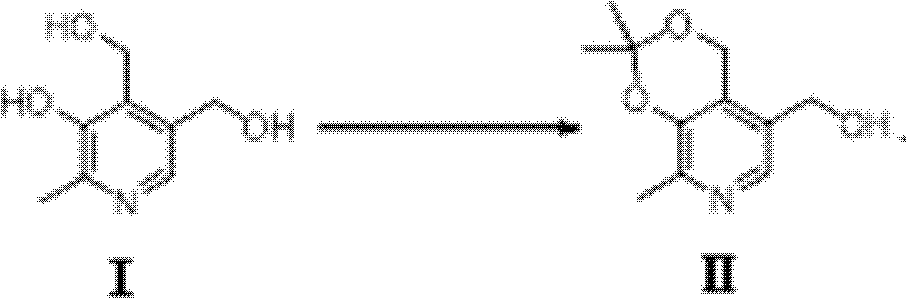
[0039] (1). Dissolve pyridoxine in acetone, add a catalytic amount of p-toluenesulfonic acid, and reflux the reaction solution overnight. After cooling, spin off the solvent under vacuum conditions, redissolve in dichloromethane, and use sodium bicarbonate aqueous solution for the solution Washing, the aqueous layer was further extracted with dichloromethane, the organic phases were combined, dried over magnesium sulfate, filtered, and further separated and purified by column chromatography to obtain (2,2,8-trimethyl-4H-[1,3]diox [4,5-c]pyridin-5-yl)methanol.
[0040]
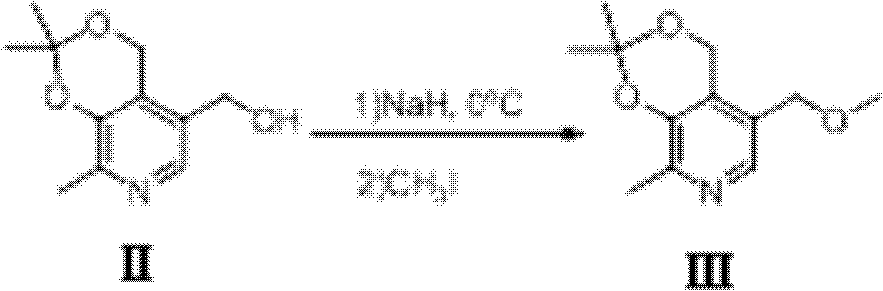
[0041] (2). Dissolve (2,2,8-trimethyl-4H-[1,3]dioxy[4,5-c]pyridin-5-yl)methanol in anhydrous tetrahydrofuran and cool to 0oC , and then sodium hydride (70% oil droplet dispersion) was added in portions. After stirring for 30 minutes, a solution of methyl iodide in tetrahydrofuran was added dropwise to the reacti...
Embodiment 2
[0054] Preparation of formula VIII 4-formyl-3-hydroxyl-5-(methoxymethyl)-1,2-lutidine salt:
[0055] (1). Preparation of (2,2,8-trimethyl-4H-[1,3]dioxy[4,5-c]pyridin-5-yl)methanol of formula II;
[0056]
[0057] Pyridoxine (4.06g, 24mmol) was dissolved in 120ml of acetone, a catalytic amount of p-toluenesulfonic acid (4.93g, 28.68mmol) was added, and the reaction solution was refluxed overnight. After cooling, spin off the solvent under vacuum, redissolve in 120ml of dichloromethane, wash the solution with aqueous sodium bicarbonate, and extract the aqueous layer with dichloromethane, combine the organic phases, dry over magnesium sulfate, filter, and use column chromatography Separation and purification gave white solid (2,2,8-trimethyl-4H-[1,3]dioxy[4,5-c]pyridin-5-yl)methanol (5.02 g, 63.3%). 1HNMR (CDCl 3 , 300MHz): 1.55(d, J=3.8Hz, 6H), 2.41(s, 3H), 4.59(d, J=5.5Hz, 2H), 4.94(s, 2H), 7.96(s, 1H).
[0058] (2). Preparation of formula III 5-(methoxymethyl)-2,2,8-trim...
Embodiment 3
[0077] N-terminal transamination of the model polypeptide leucine-glutamic acid-tryptophan-glycine-alanine (LEWGA).
[0078] To the in situ generated pyridoxal derivative of formula VIII was added 500 μL of 100 mM phosphate buffered saline. The solution was mixed well, and the solution was adjusted to pH=6.5 with 1M sodium hydroxide and 1M hydrochloric acid. Then an equal volume of 2 mM peptide (LEWGA) solution was added, the solution was remixed and the pH was adjusted to 6.5. The reaction was stirred at room temperature and followed by RP-HPLC (C18 column, UV detection at 215 nm and 280 nm) and LC-MS. The reaction time was 27 hours and the yield was 88%. LC-MS: (CH 3 ) 2 CHCH2 COCO-Glu-Trp-Gly-Ala-OH (theoretical 573.2, actual [M+H] + 574.2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com