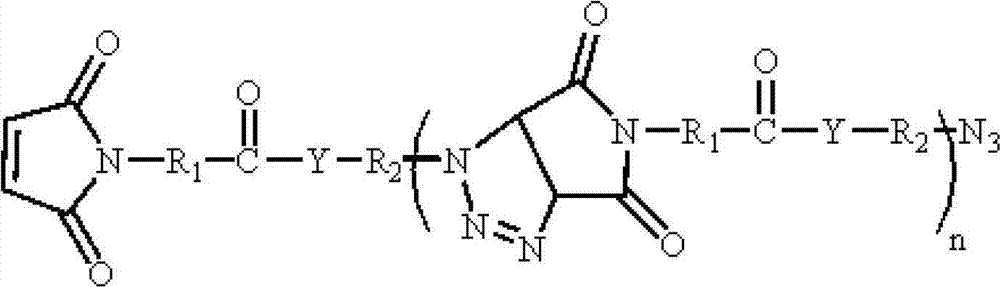
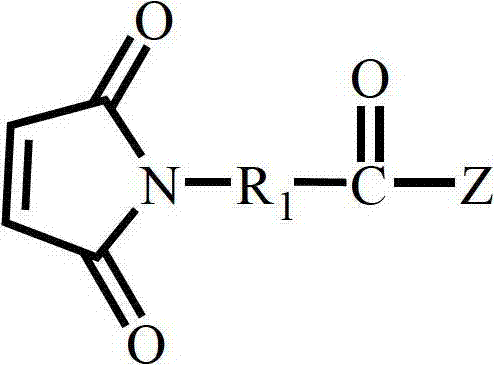
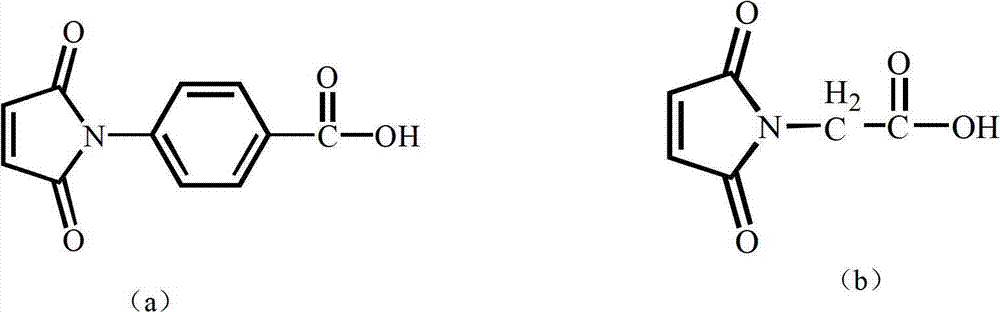Polymaleimide polymer as well as preparation method and application of polymaleimide polymer
A technology of polymaleimide and maleimide, which is applied in the field of polymer material synthesis to achieve good heat resistance, ensure singleness, and good bonding performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Synthesis of Substance A
[0056] Add 0.1 mol of 4-(2,5-dioxo-4,5-dihydro-1H-pyrrol-1-yl)benzoyl chloride, 0.1 mol of triethylamine and 0.1 mol of chloroethanol into 2 mol of dichloromethane, React at 0°C for 2 hours to obtain reaction solution B; wash reaction solution B with 2ml of saturated aqueous sodium bicarbonate solution 3 times, then wash with 2ml of 1M hydrochloric acid solution for 3 times, then wash reaction solution B with distilled water until neutral, and then wash with anhydrous Magnesium sulfate was dried, filtered, and the filtrate was taken, concentrated to obtain a concentrated solution, and the concentrated solution was precipitated in 20ml of ether to obtain substance A;
[0057] The structural formula of the reaction is as follows:
[0058]
[0059] (2) Preparation of polymaleimide polymer:
[0060] Add 0.1 mol of substance A in step (1) and 0.1 mol of sodium azide to 2 mol of dimethyl sulfoxide at the same time, and react at 20°C for 6 h...
Embodiment 2
[0064] (1) Synthesis of Substance A
[0065] Add 0.1mol 4-(2,5-dioxo-4,5-dihydro-1H-pyrrol-1-yl)benzoic acid, 0.2mol sodium p-toluenesulfonate and 0.2mol bromoethanol to 10mol tetrahydrofuran, React at 25°C for 24 hours to obtain reaction solution B; wash reaction solution B with 2ml of saturated aqueous sodium bicarbonate solution 5 times, then wash reaction solution B with 2ml of 1M hydrochloric acid solution for 5 times, then wash reaction solution B with distilled water until neutral, and then use Dry over magnesium sulfate, filter, take the filtrate, concentrate to obtain a concentrated solution, and precipitate the concentrated solution in 3ml of n-hexane to obtain substance A;
[0066] The structural formula of the reaction is as follows:
[0067]
[0068] (2) preparation of polymaleimide polymer:
[0069] Add 0.1 mol of substance A in step (1) and 1 mol of sodium azide to 20 mol of dimethylformamide at the same time, react at 100°C for 72 hours to obtain a reactio...
Embodiment 3
[0073] (1) Synthesis of Substance A
[0074] Add 0.1mol 2-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl) acetyl chloride, 0.15mol pyridine and 0.15mol chloropropanol to 5mol toluene and react at 15°C After 14 hours, the reaction solution B was obtained; the reaction solution B was washed 4 times with 2 ml of saturated aqueous sodium bicarbonate solution, and then 4 times with 2 ml of 1M hydrochloric acid solution, and then the reaction solution B was washed with distilled water until neutral, and then washed with anhydrous magnesium sulfate Drying, filtering, taking the filtrate, concentrating to obtain a concentrated solution, and precipitating the concentrated solution in 10ml of cyclohexane to obtain substance A;
[0075] The structural formula of the reaction is as follows:
[0076]
[0077] (2) Preparation of polymaleimide polymer:
[0078] 0.1 mol of substance A in step (1) and 0.5 mol of sodium azide were added to 10 mol of tetrahydrofuran at the same time, and reacted at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com