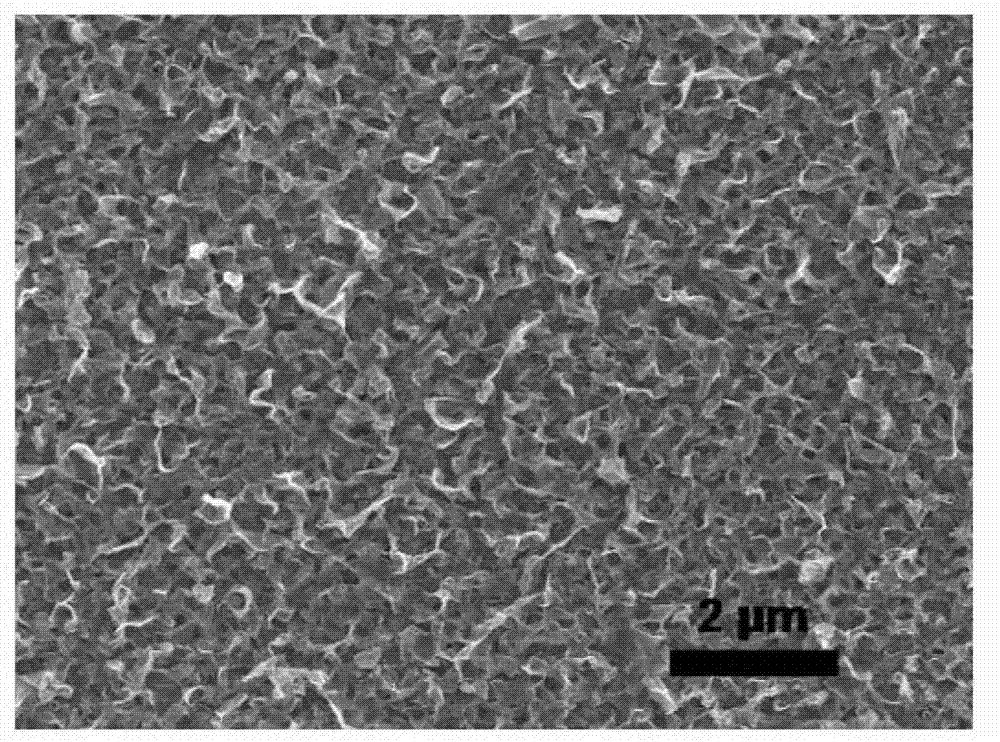
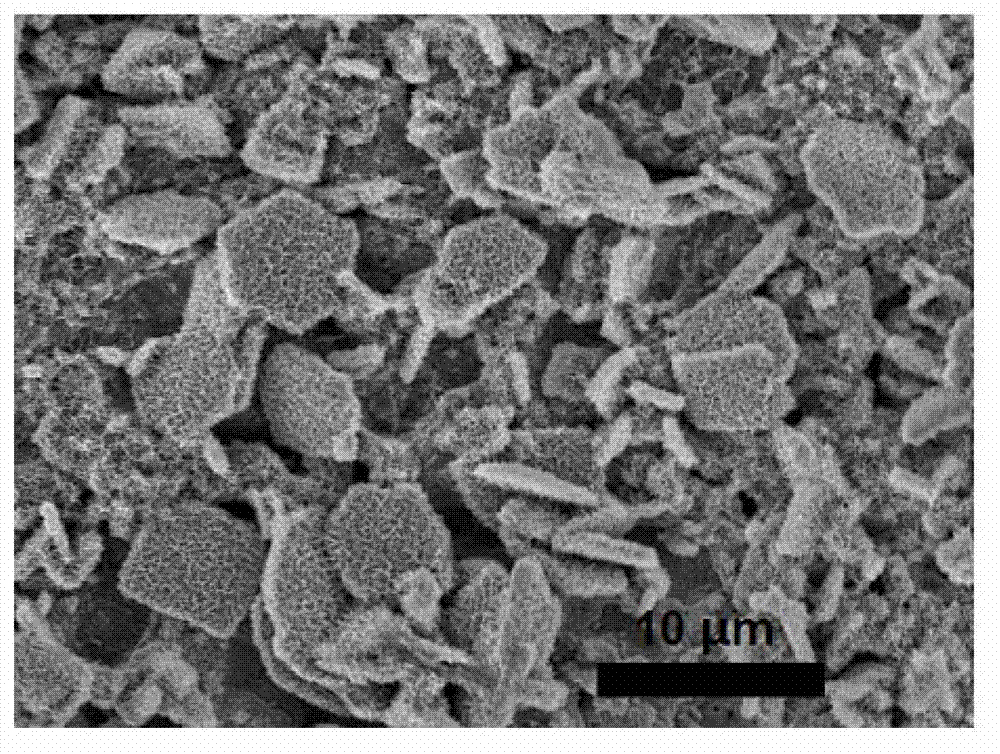
Anodization two-step preparation method of cuprous oxide nanosheet powder material
A technology of anodic oxidation and nano flakes, applied in the direction of anodic oxidation, etc., can solve the problems of additive removal, high energy consumption, complex process, etc., and achieve the effect of short experiment period, low energy consumption and obvious photoelectric response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 10.7g (0.2mol) NH 4 Cl was dissolved in 1L deionized water to prepare a buffer solution, and the pH value was adjusted to 6 with dilute HCl solution. Cut the high-purity copper foil into small pieces of 4cm×3cm. After cleaning, grinding and polishing, stick the back and surroundings with waterproof tape so that the exposed area is 4cm 2 (2cm x 2cm). Fix the copper foil (anode) and graphite electrode (cathode) on both sides of the electrolytic cell, and connect them directly to the two poles of the DC power supply with wires. Pour the electrolyte into the electrolytic cell, adjust the DC power supply to the constant current mode, and set the current to 5mA / cm 2 , starts to oxidize at room temperature. After 10 minutes, the power was cut off, and the sample was washed and dried to obtain a dark red CuCl film. Immerse the CuCl film in 0.3% H 2 o 2 The solution was placed under natural light to react for 30 minutes, and after taking it out, it was washed and dried to ...
Embodiment 2
[0027] 10.7g (0.2mol) NH 4 Cl was dissolved in 1L deionized water to prepare a buffer solution, and the pH value was adjusted to 9 with dilute HCl solution. Cut the high-purity copper foil into small pieces of 4cm×3cm. After cleaning, grinding and polishing, stick the back and surroundings with waterproof tape so that the exposed area is 4cm 2 (2cm x 2cm). Fix the copper foil (anode) and graphite electrode (cathode) on both sides of the electrolytic cell, and connect them directly to the two poles of the DC power supply with wires. Pour the electrolyte into the electrolytic cell, adjust the DC power supply to the constant current mode, and set the current to 5mA / cm 2 , starts to oxidize at room temperature. Cut off the power after 10 minutes, and the sample was washed and dried to obtain a blue-green loose copper oxychloride film. Immerse the sample in 0.3% H 2 o 2 The solution was placed under natural light to react for 30 minutes, and after taking it out, it was washed...
Embodiment 3
[0029] 10.7g (0.2mol) NH 4 Cl was dissolved in 1L deionized water to prepare a buffer solution, and the pH value was adjusted to 9 with dilute HCl solution. Cut the high-purity copper foil into small pieces of 4cm×3cm. After cleaning, grinding and polishing, stick the back and surroundings with waterproof tape so that the exposed area is 4cm 2 (2cm x 2cm). Fix the copper foil (anode) and graphite electrode (cathode) on both sides of the electrolytic cell, and connect them directly to the two poles of the DC power supply with wires. Pour the electrolyte into the electrolytic cell, adjust the DC power supply to the constant current mode, and set the current to 5mA / cm 2 , starts to oxidize at room temperature. Cut off the power after 10 minutes, and the sample was washed and dried to obtain a blue-green loose copper oxychloride film. Immerse the sample in 1% H 2 o 2In the solution, put it under a xenon lamp light source to react for 60 minutes, take it out, wash and dry it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com