High specific activity xylosidase Xyl52B8 and gene and application thereof
A technology of live xylosidase and xylosidase, applied in high specific activity xylosidase Xyl52B8 and its gene and application field, can solve the problems of poor thermal stability and unsatisfactory, and achieve good thermal stability and high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Cloning of Alicyclobacillus sp.B8 Xylosidase Encoding Gene xyl52
[0079] Extract Genomic DNA of Alicyclobacillus sp.B8: Extract the bacterial cells cultured in liquid for 1 day with a bacterial extraction kit, add appropriate amount of TE to dissolve, and store at -20°C for later use.
[0080] The degenerate primers P1 and P2 were designed and synthesized according to the conserved (GNEMDG and DEWNVW) sequences of the xylosidase genes of the 52nd family
[0081] P1: 5'-GGNAAYGARATGGAYGG-3';
[0082] P2: 5'-GGAEITTYD SLDVSLGQ-3'.
[0083] Alicyclobacillus sp.B8 total DNA was used as template for PCR amplification. The PCR reaction parameters were: denaturation at 94°C for 5 min; then denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec, extension at 72°C for 1 min, and after 30 cycles, incubation at 72°C for 10 min. A fragment of about 2 kb was obtained, which was recovered and connected with the pEASY-T3 vector and sent to Sanbo Biotechnology Co.,...
Embodiment 2
[0088] The preparation of embodiment 2 recombinant xylosidase
[0089] The expression vector pPET-30a was subjected to double enzyme digestion (Ecoli I+Not I), and the gene xyl52B8 encoding xylosidase was double enzyme digested (Ecoli I+Not I) at the same time, and the gene fragment encoding mature xylosidase was cut out and expressed The vector pPET-30a was ligated to obtain the recombinant plasmid pPET-xyl52B8 containing the xylosidase gene xyl52B8 and transformed into Escherichia coli BL21(DE3) to obtain the recombinant Escherichia coli BL21 / xyl52B8.
[0090] The BL21 strain containing the recombinant plasmid was inoculated in 300mL LB culture medium, shaken at 220rpm at 37°C for 3h, and then induced with a final concentration of 0.6mM IPTG at 30°C for 4h. The expression level of recombinant xylosidase was 69.86U / mL. The results of SDS-PAGE showed that the recombinant xylosidase was expressed in Escherichia coli. The specific activity of recombinant xylan is 200.7U / mg.
Embodiment 3
[0091] The activity analysis of embodiment 3 recombinant xylosidases
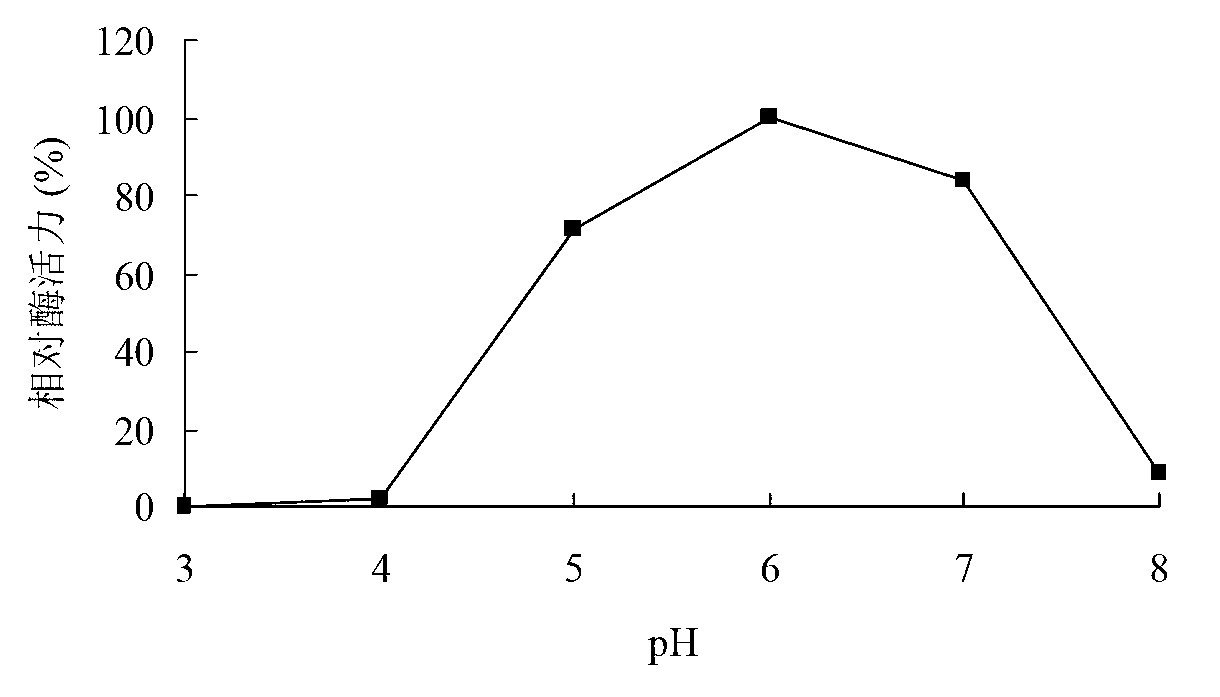
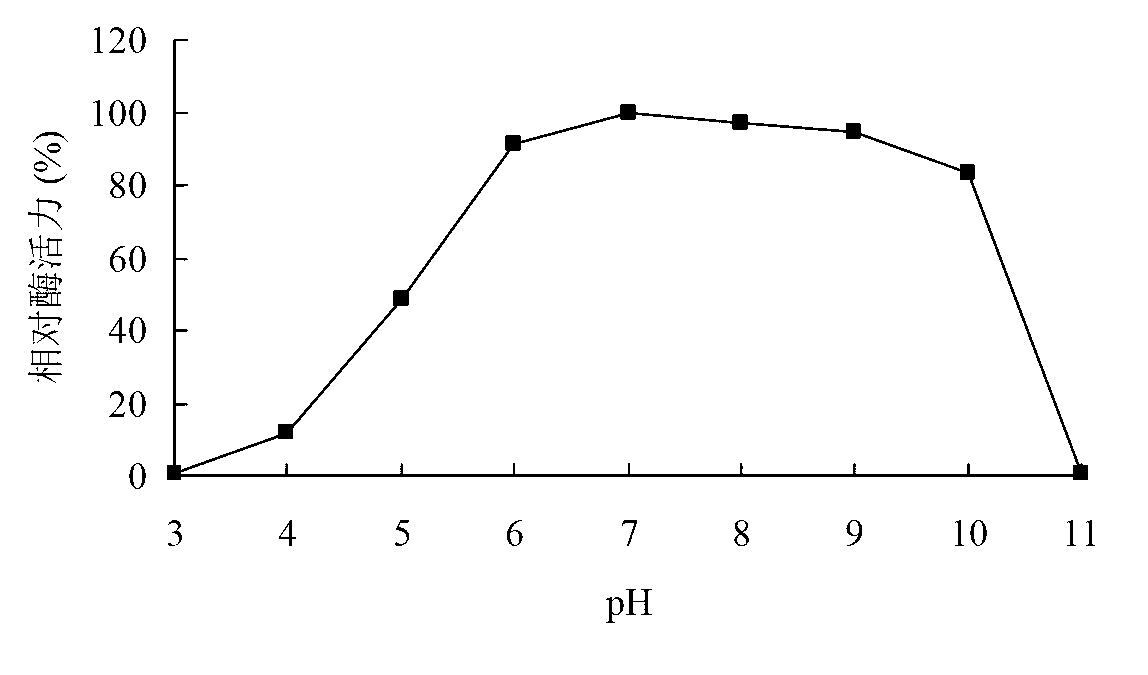
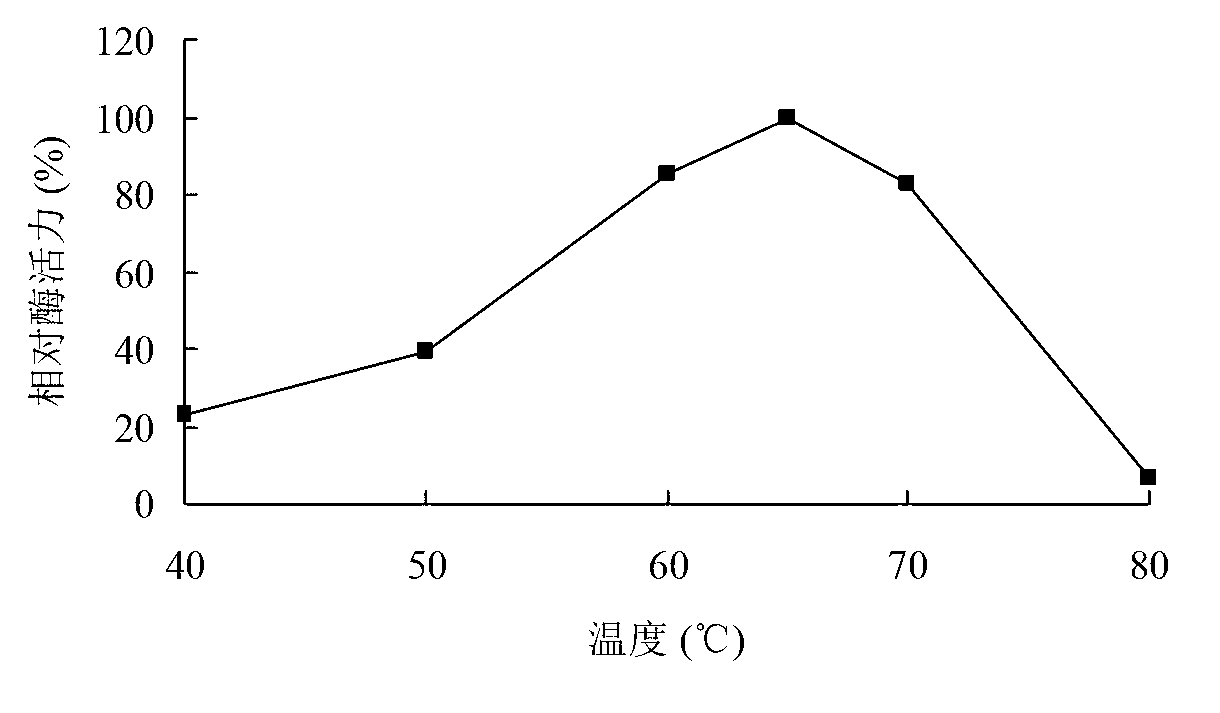
[0092] Determination of xylosidase activity: measure the amount of p-nitrophenol produced by enzymatic hydrolysis of substrate pNPX at 405 nm. Reaction steps: Mix 250 μL of 2mM pNPX substrate with 150 μL of buffer, add 100 μL of appropriately diluted enzyme solution, react at 50°C for 10 min, add 1.5 mL of 1M Na2CO3 to terminate the reaction, and measure the OD value at 405 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com