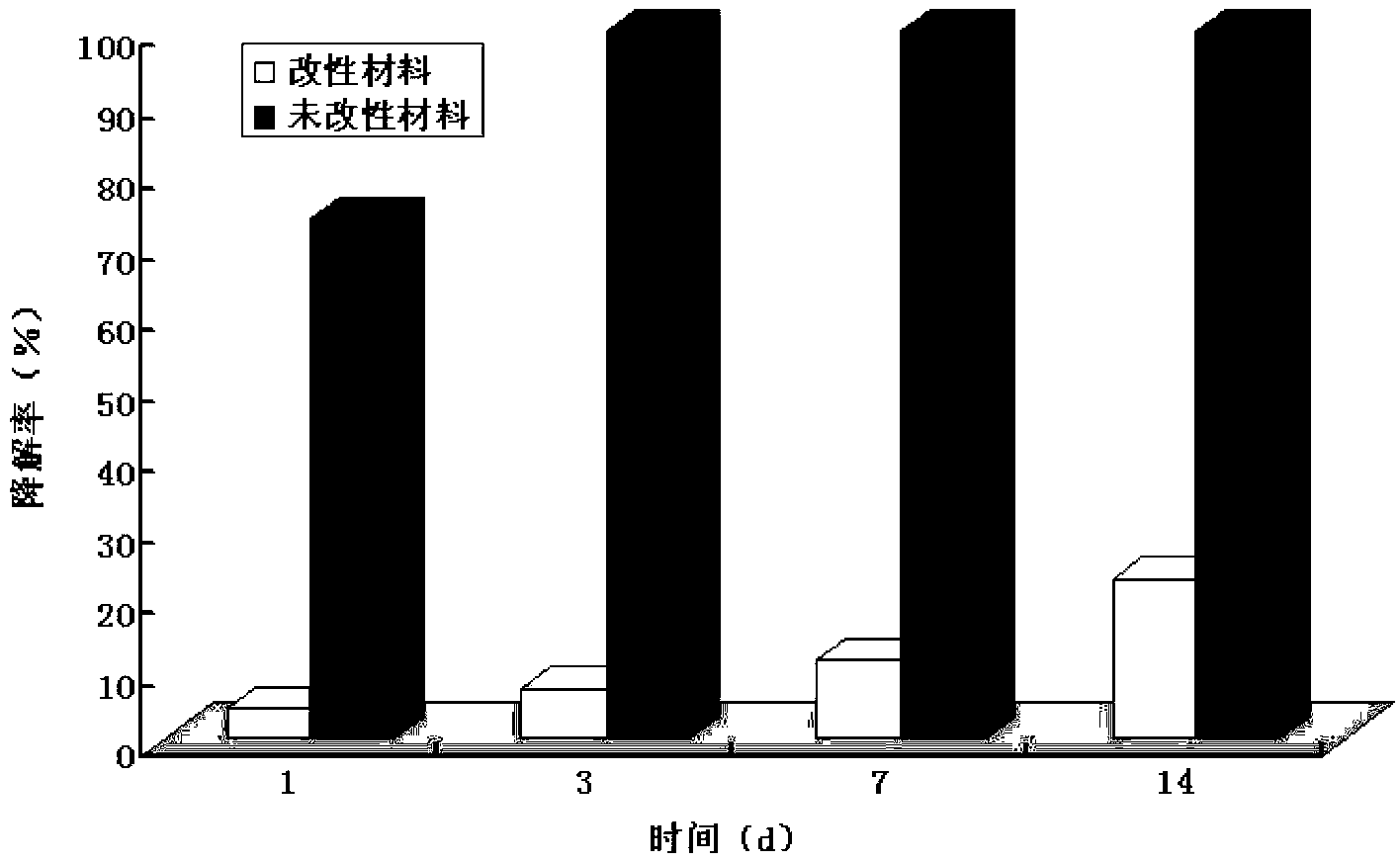
Modified rat accellular spinal cord bracket material and preparation method thereof
A scaffold material and decellularization technology, which is applied in the field of tissue engineering biomaterials, can solve the problems of high cytotoxicity, poor structural stability, and stiff texture of materials, and achieve the effects of broad application prospects, high porosity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] 1. Preparation of modified rat acellular spinal cord scaffold material
[0049] (1) Laboratory animals and equipment
[0050] Whole rat spinal cord frozen at -70°C (purchased from Experimental Animal Center of Daping Hospital, Third Military Medical University), sodium pentobarbital (P3761, Sigma), sodium chloride (51024060, Sinopharm Group), TritonX-100 (ST795) , Biyuntian), Sodium Deoxycholate (D001601, Amresco), Genipin (6902-77-8, Linchuan Zhixin Biological Technology Co., Ltd.), Glutaraldehyde (111-30-8, Shanghai Ziyi Reagent Factory ), phosphate buffer solution (PBS) (AR0030, Boster), surgical instruments (Shanghai Medical Instrument Factory), ultrapure water purifier (Millipore, USA), freeze dryer (Thermo Savant Modulyo D, USA), desktop Constant temperature oscillator (THZ-22, Jiangsu Taicang Experimental Equipment Factory), refrigerator (Midea), -70℃ deep low temperature refrigerator (Sanyo, Japan).
[0051] (2) Method
[0052] Preparation of modified rat acellular sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com