Improved method for preparing 4-phenyl-cyanophenyl
A technology of phenyl and benzonitrile, applied in the field of preparing 4-phenyl-benzonitrile, which can solve the problems of difficult operation, difficult handling, increased difficulty of 4-phenyl-benzonitrile and equipment investment, and achieve simplified post-processing Steps, the effect of reducing reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
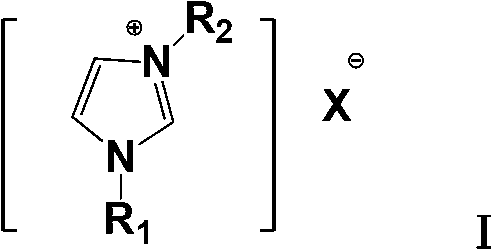
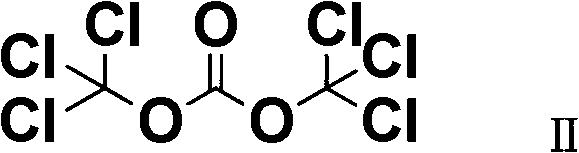
[0029] Add 15.4g of biphenyl, 4oomL of solvent (chlorobenzene or o-dichlorobenzene) into a suitable reactor, and add 1-methyl-3-n-butylimidazolium chloride ionic liquid [BmimCl-ACl 3 ]32.0g, stirred, dripped 21.8g of trichloroacetyl chloride, controlled the reaction temperature at -10°C to 50°C, stirred, and detected by liquid chromatography. Organic phase, organic solvent (chlorobenzene or o-dichlorobenzene) extracts the ionic liquid phase (water phase), combines the organic phases, and repeatedly washes the organic phase to obtain a light yellow Clear solution.
[0030] Into the light yellow transparent solution containing 1-trichloroacetyl-4-phenyl-benzene, directly feed ammonia gas or add ammonia water, control the temperature at -10°C to 50°C, and detect the intermediate (1 -Trichloroacetyl-4-phenyl-benzene) When the content is lower than 5%, stop feeding ammonia gas or add concentrated ammonia water. A solvent (chlorobenzene, dichloroethane or dichloromethane) was adde...
Embodiment 2
[0035] Add 154g of biphenyl, 4L of solvent (dichloroethane or dichloromethane) into a suitable reactor, add 1-methyl-3-isopropylimidazolium ionic liquid [BmimCl-ACl 3 ] 320g, stirred, dripped 218g of trichloroacetyl chloride, controlled the reaction temperature to be -10°C to 50°C, stirred, and detected by liquid chromatography. , an organic solvent (dichloroethane or dichloromethane) extracts the ionic liquid phase (aqueous phase), combines the organic phases, and repeatedly washes the organic phases to obtain a light yellow transparent solution.
[0036] Into the above-mentioned light yellow transparent solution containing 1-trichloroacetyl-4-phenyl-benzene, directly pass ammonia gas or add ammonia water, control the temperature at -10°C to 50°C, and detect the intermediate (1- When the content of trichloroacetyl-4-phenyl-benzene) is lower than 5%, stop feeding ammonia gas or add concentrated ammonia water. A solvent (chlorobenzene or o-dichlorobenzene) is added to the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com