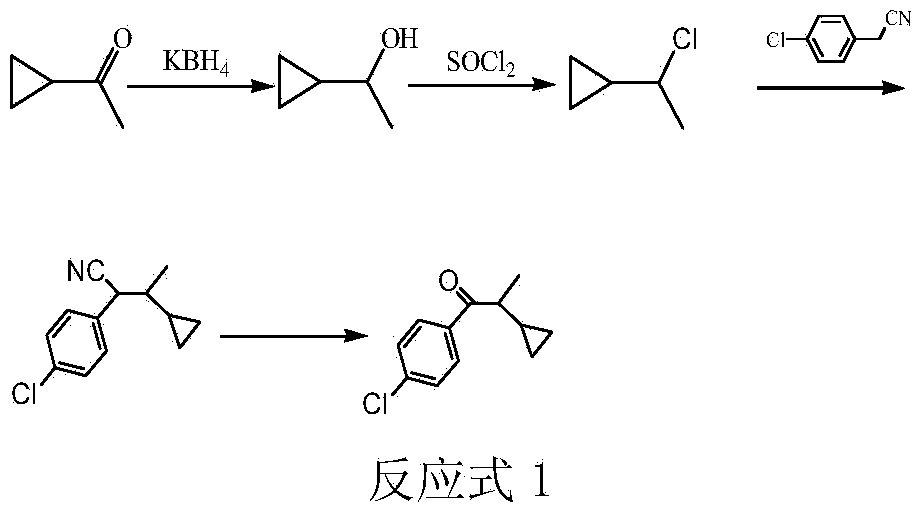
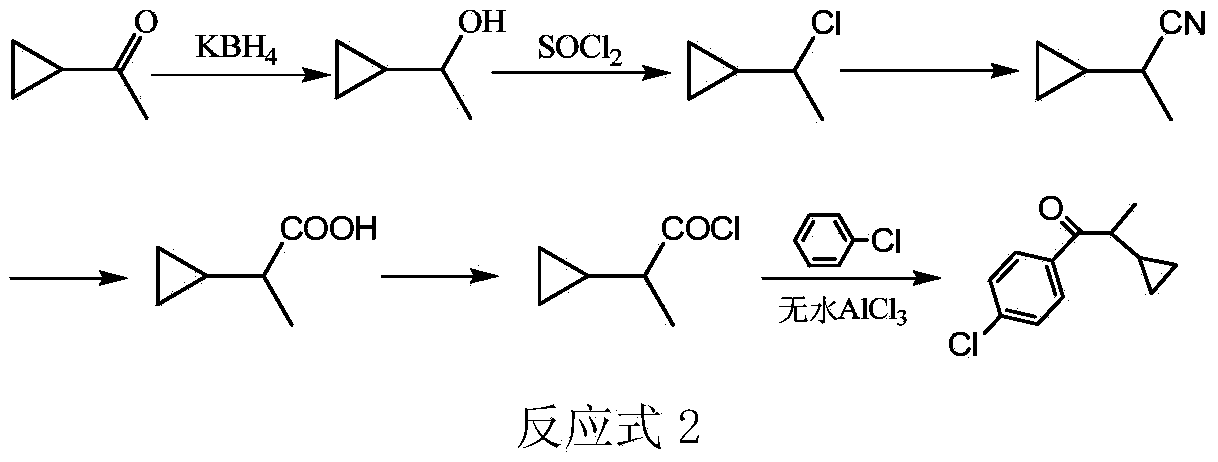
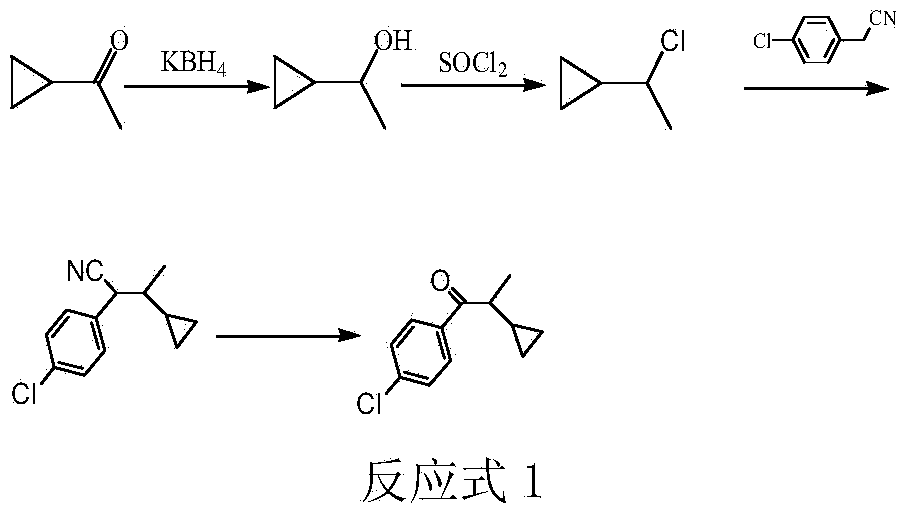
The preparation method of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone
A technology of chlorophenyl and cyclopropyl, which is applied in the preparation of carbon-based compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of unsuitability for industrial scale production, unsafe magnesium powder reduction operation, and high cost of raw materials , to achieve the effects of low production cost, high product yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 8.4g (0.1mol) of cyclopropylmethyl ketone, 2.2g (0.04mol) of potassium borohydride, 42g of methanol, and 42g of water into a 250mL three-necked flask with a stirring and reflux tube, and react at room temperature for 4h. After the reaction was finished, neutralize with 6N hydrochloric acid, extract and separate the liquids with ethyl acetate, dry the organic phase by adding anhydrous sodium sulfate, and remove the solvent to obtain 8.6g of 1-cyclopropylethanol with a purity of 99% (gas chromatography), and a yield of 99% (based on Cyclopropylmethyl ketone).
[0016] Add 8.6g of 1-cyclopropylethanol to a 100mL three-necked flask, slowly drop in 14.3g (0.12mol) of thionyl chloride at 10°C, add it in 30min, and react at 10°C for 4h. After the reaction was completed, the excess thionyl chloride and HCl were removed under reduced pressure, the reaction solution was washed twice with 20 mL of water, and dried by adding anhydrous sodium sulfate to obtain 10.3 g of 1-cyclop...
Embodiment 2
[0020] Add 8.4g (0.1mol) of cyclopropylmethyl ketone, 2.2g (0.04mol) of potassium borohydride, 21g of methanol, and 21g of water into a 100mL three-necked flask with a stirring and reflux tube, and react at room temperature for 4h. After the reaction was finished, neutralize with 6N hydrochloric acid, extract and separate the liquids with ethyl acetate, dry the organic phase by adding anhydrous sodium sulfate, and remove the solvent to obtain 8.2g of 1-cyclopropylethanol with a purity of 98% (gas chromatography) and a yield of 93.4% (based on Cyclopropylmethyl ketone).
[0021] Add 8.2g of 1-cyclopropylethanol to a 250mL three-necked flask, slowly drop in 13.1g (0.11mol) of thionyl chloride at 10°C, add it in 1h, and react at 10°C for 4h. After the reaction was completed, the excess thionyl chloride and HCl were removed under reduced pressure, the reaction solution was washed twice with 20 mL of water, and dried by adding anhydrous sodium sulfate to obtain 9.5 g of 1-cycloprop...
Embodiment 3
[0025] Add 8.4g (0.1mol) of cyclopropylmethyl ketone, 2.2g (0.04mol) of potassium borohydride, 34g of methanol, and 34g of water into a 250mL three-necked flask with a stirring and reflux tube, and react at room temperature for 4h. After the reaction was finished, neutralize with 6N hydrochloric acid, extract and separate the liquids with ethyl acetate, dry the organic phase by adding anhydrous sodium sulfate, and remove the solvent to obtain 8.4g of 1-cyclopropylethanol with a purity of 98.5% (gas chromatography) and a yield of 96.2% (based on Cyclopropylmethyl ketone).
[0026] Add 8.4g of 1-cyclopropylethanol to a 100mL three-necked flask, slowly drop in 14.3g (0.12mol) of thionyl chloride at 10°C, add it in 30min, and react at 10°C for 4h. After the reaction was over, the excess thionyl chloride and HCl were removed under reduced pressure, the reaction solution was washed twice with 20 mL of water, and dried by adding anhydrous sodium sulfate to obtain 9.9 g of 1-cycloprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com