Halogen-free phosphonate flame-retardant modified waterborne polyurethane coating agent and preparation method thereof
A technology of water-based polyurethane and halophosphonate, which is applied in textiles, papermaking, fiber treatment, etc., can solve the problems that affect the application range of flame retardants and are prone to cracking, and achieve the effect of small impact and high flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
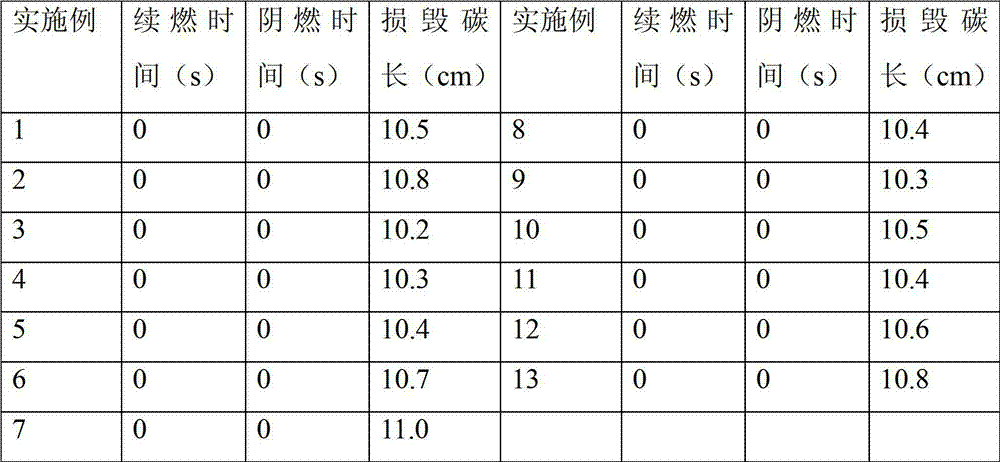
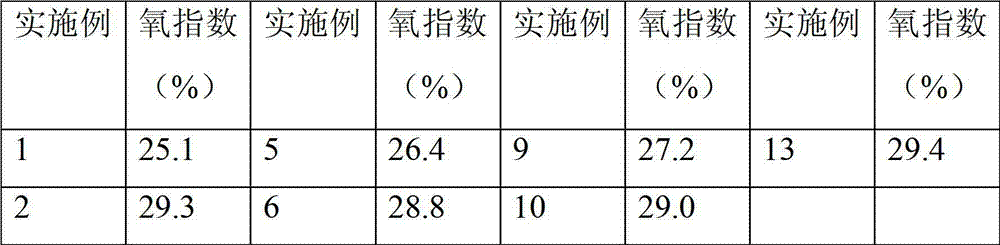
Examples
Embodiment 1
[0026] Under nitrogen protection atmosphere and stirring conditions, 3.07g of 2-phenoxy(3-hydroxy)phenylphosphine oxide (BPHPPO) with a hydroxyl value of 327mgKOH / g, 9.02g of polyether polyol with a hydroxyl value of 112mgKOH / g Alcohol N210 and 8.46 g of isophordione diisocyanate IPDI were placed in a flask, and a partial substitution reaction was carried out at a temperature of 75° C., and the reaction was stirred for 1.5 h.
[0027] Add 1.25g of chain extender dimethylolpropionic acid DMPA into the flask for chain extension, and react at 70°C for 3h. In order to prevent the system from being too viscous, add an appropriate amount of acetone to the above-mentioned flask to adjust the viscosity.
[0028] Cool the reaction intermediate product in the flask to below 40°C, add 1.08g of salt-forming agent triethylamine (TEA) and neutralize for 10 minutes to obtain a polyurethane prepolymer partially substituted with phosphonate-modified flame-retardant polyol.
[0029] Add 35.77 ...
Embodiment 2
[0031] Under nitrogen protection atmosphere and stirring conditions, the hydroxyl value of 4.81g is 455mgKOH / g. The structural formula is: The phosphonate-modified polyol, 8.90g polycastor oil adipate polyol with a hydroxyl value of 93mgKOH / g, and 12.45g toluene diisocyanate TDI were placed in a flask, and stirred and reacted at 70°C for 2h.
[0032] Add 3.42g chain extender containing sulfonate-type chain extender 1,4-butanediol-2-sodium sulfonate into the flask, and react at 65°C for 4h. In order to prevent the system from being too viscous, add an appropriate amount of acetone to the above-mentioned flask to adjust the viscosity.
[0033] Cool the reaction intermediate product in the flask to below 40°C, add 2.46g of salt-forming agent ammonia water and neutralize it for 10 minutes to obtain a prepolymer.
[0034] Add 75.31 g of deionized water into the flask, and stir vigorously for 35 minutes at a stirring speed of 850 r / min, so that the prepolymer is uniformly disperse...
Embodiment 3
[0036] Under nitrogen protection atmosphere and stirring conditions, the hydroxyl value of 6.50g is 496mgKOH / g. The structural formula is: The phosphonate-modified polyol, 8.52g of polyether polyol PPG with a hydroxyl value of 105mgKOH / g, and 7.30g of diphenylmethane diisocyanate MDI were placed in a flask, and stirred and reacted at 65°C for 2h.
[0037] Add 0.95g of cationic chain extender N-methyldiethanolamine into the flask, and react at 60°C for 4h. In order to prevent the system from being too viscous, add an appropriate amount of acetone to the above-mentioned flask to adjust the viscosity.
[0038] Cool the reaction intermediate product in the flask to below 40°C, add 0.92g of salt-forming agent acetic acid and neutralize it for 10 minutes to obtain a prepolymer.
[0039] Add 57.23 g of deionized water into the flask, and stir vigorously for 30 minutes at a stirring speed of 900 r / min, so that the prepolymer is uniformly dispersed in the deionized water, and the ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com