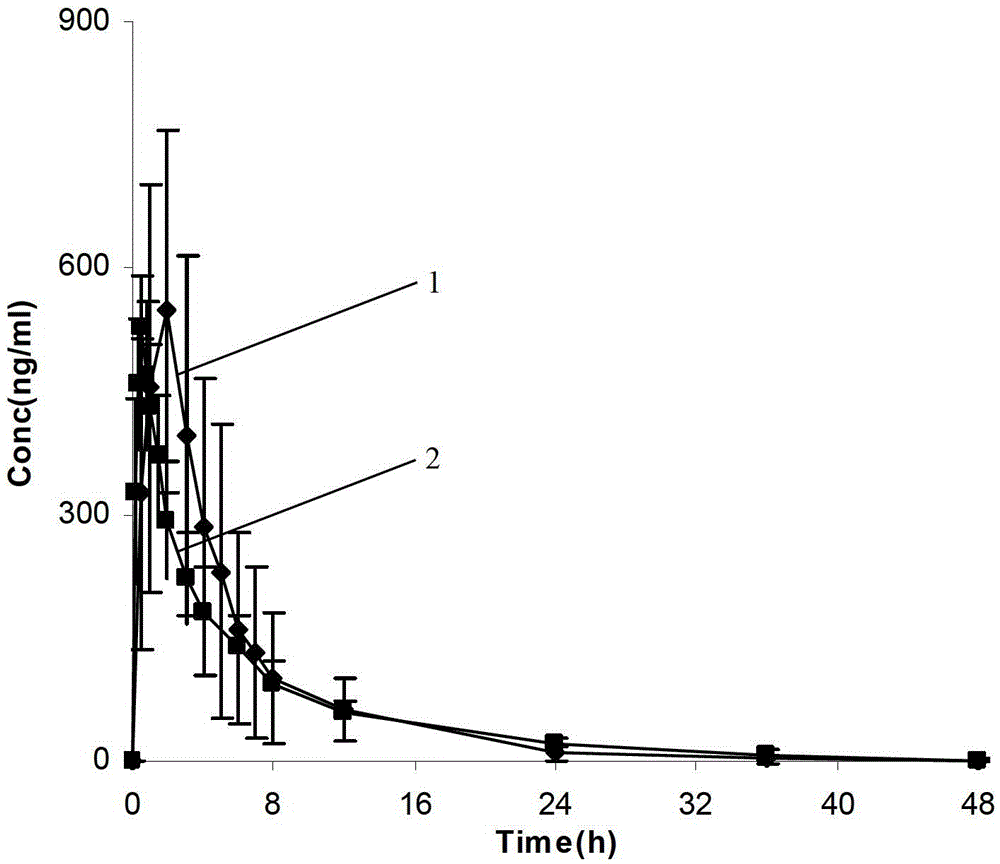
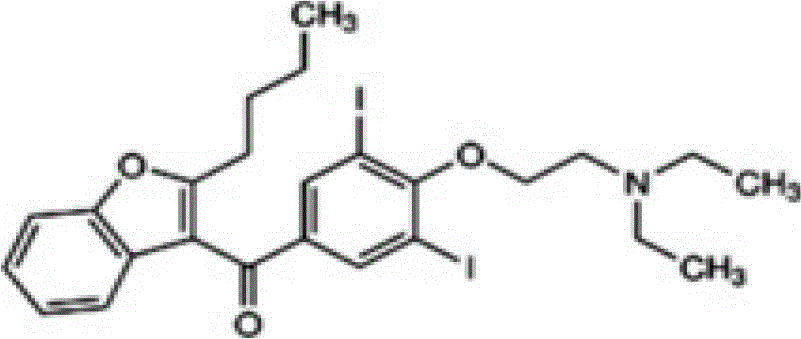
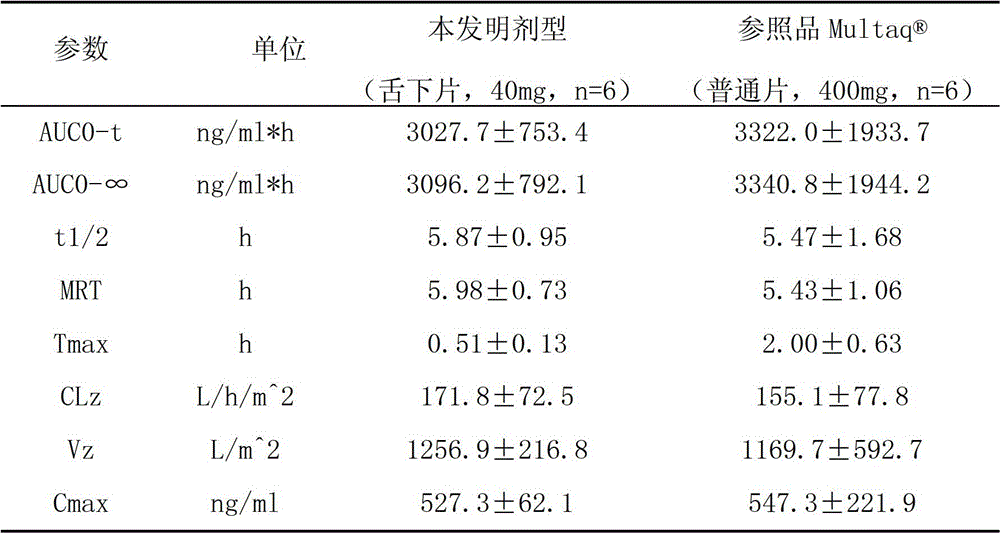
A dronedarone hydrochloride pharmaceutical composition and preparation method thereof
A technique for dronedarone hydrochloride and its composition, which is applied in the field of dronedarone hydrochloride pharmaceutical composition and preparation, can solve the problems of complex manufacturing process, high cost, poor stability, etc., and achieve improved bioavailability and rapid onset of effect , strong penetration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation prescription: dronedarone hydrochloride 5g, lactose 40g, mannitol 40g, cross-linked polyvinylpyrrolidone 5g, lactic acid 0.5g, stevioside 5g, polyethylene glycol 4000lg, polyvinylpyrrolidone 2g.
[0044] The specific preparation method is as follows: dry, pulverize and sieve the main drug, filler lactose and mannitol, disintegrant cross-linked polyvinylpyrrolidone, flavoring agent stevioside and lubricant polyethylene glycol 4000; Dissolve the binder polyvinylpyrrolidone and the antacid agent lactic acid in an appropriate amount of water to make a binder solution; mix the pretreated main drug with fillers, flavoring agents and disintegrants, and use the prepared binder Soft material made from solvent solution, granulated with a 20-mesh sieve; dried at 40°C until moisture content ≤ 2%; added lubricant to the obtained dry granules, blended, and finally compressed to obtain Dronedarone Hydrochloride Sublingual Tablets .
Embodiment 2
[0046] Preparation prescription: dronedarone hydrochloride 40g, microcrystalline cellulose-mannitol 40g, cross-linked polyvinylpyrrolidone 5g, citric acid 2g, stevioside 2g, polyethylene glycol 40000.5g, polyvinylpyrrolidone 5g.
[0047] The specific preparation method is as follows: dry the main drug, fill microcrystalline cellulose-mannitol, disintegrant cross-linked polyvinylpyrrolidone, corrective agent stevioside and lubricant polyethylene glycol 4000, dry, pulverize, and sieve. Treatment; dissolve the binder polyvinylpyrrolidone and the antacid agent citric acid in an appropriate amount of water to make a binder solution; mix the pretreated main ingredient with fillers, flavoring agents and disintegrants, and use the prepared A good binder solution made of soft material, granulated with a 20-mesh sieve; dried at 50°C until the moisture content ≤ 2%; added lubricant to the obtained dry granules, mixed, and finally compressed to obtain dronedar hydrochloride Sublingual aug...
Embodiment 4
[0052] Preparation prescription: dronedarone hydrochloride 20g, microcrystalline cellulose-mannitol 30g, cross-linked polyvinylpyrrolidone 1g, citric acid 1.5g, stevioside 1g, polyethylene glycol 40000.3g, polyvinylpyrrolidone 1g.
[0053] The specific preparation method is as follows: dry, pulverize and sieve the main ingredient, filler sugar powder, mannitol, sodium carboxymethyl starch, flavoring agent steviol glycoside and lubricant magnesium stearate; The agent polyvinylpyrrolidone and the antacid agent citric acid are dissolved in an appropriate amount of water to prepare an adhesive solution; the pretreated main drug is mixed with fillers, flavoring agents and disintegrants, and the prepared adhesive solution is used The soft material is granulated with a 20-mesh sieve; dried at 60°C until the moisture content is ≤2%; the lubricant is added to the obtained dry granules for total blending, and finally compressed to obtain dronedarone hydrochloride sublingual tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com