Preprocessing method for removing nanoscale iron-bearing substance Kelvin balance disturbance
A nano-scale, pretreatment technology, applied in the preparation of test samples, etc., can solve the problems of inaccurate determination of organic matter content in wastewater, inability to remove dissolved iron colloids, and interference in measurement results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] When the methyl orange content was determined after the methyl orange-containing wastewater was treated by adsorption of iron-containing oxides, analytically pure aluminum sulfate was added to eliminate the kevin equilibrium interference test of nano-scale iron-containing substances.
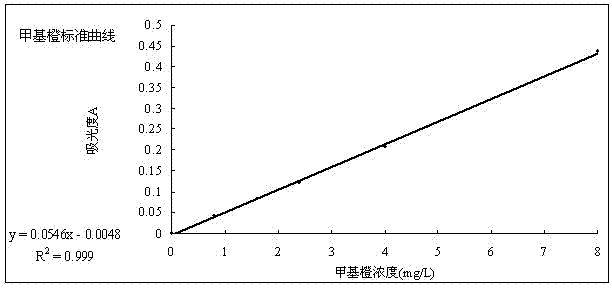
[0021] 1. Draw a methyl orange standard curve
[0022] 1.1 Weigh 0.02g of methyl orange powder with an analytical balance, dissolve it, and make 1L of 20mg / l methyl orange solution in a volumetric flask, and use the gradient dilution method to configure 0, 0.8, 1.6, 2.4, 4, 4.8, 8mg / l l standard solution.
[0023] 1.2 Use 1.6mg / l methyl orange solution to scan on the UV-3100PC ultraviolet-visible spectrophotometer, and the best absorption wavelength is 476.2nm.
[0024] 1.3 Measure the absorbance of the solution prepared in 1.1 at a wavelength of 476.2nm, and draw a standard curve. The results are shown in Table 1 and figure 1 .
[0025] Table 1
[0026] Methyl orange standard curve d...
Embodiment 2
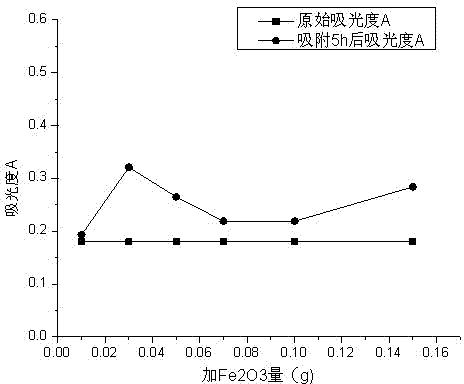
[0073] In the absence of aluminum sulfate, add Fe 2 o 3 The test results of adsorbing methyl orange, pentachlorophenol or phenol are respectively Table 9, figure 2 ; Table 10, image 3 ; Table 11, Figure 4 .
[0074] Table 9 with Fe 2 o 3 Adsorption of methyl orange, without adding aluminum sulfate flocculation to eliminate interference
[0075]
[0076] Table 10 with Fe 2 o 3 Adsorption of pentachlorophenol without adding aluminum sulfate flocculation to eliminate interference
[0077]
[0078]
[0079] Table 11 with Fe 2 o 3 Adsorption of phenol without adding aluminum sulfate flocculation to eliminate interference
[0080]
Embodiment 3
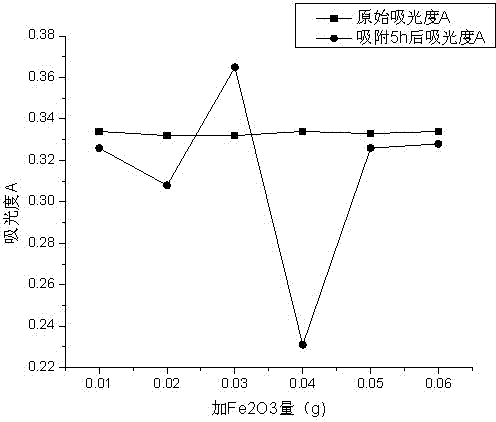
[0082] Add aluminum sulfate to adjust the pH to 7-7.5, flocculate and precipitate to eliminate interference, add Fe 2 o 3 The test results of adsorbing methyl orange, pentachlorophenol or phenol are respectively in Table 12, Figure 5 ; Table 13, Figure 6 ; Table 14, Figure 7 .
[0083] Table 12 with Fe 2 o 3 Adsorb methyl orange, add aluminum sulfate to adjust pH to 7-7.5, flocculate and precipitate to eliminate interference
[0084]
[0085] Table 13 Fe 2 o 3 Adsorb pentachlorophenol, add aluminum sulfate to adjust pH to 7-7.5, flocculate and precipitate to eliminate interference
[0086]
[0087] Table 14 with Fe 2 o 3 Adsorb phenol, add aluminum sulfate to adjust pH to 7-7.5, flocculate and precipitate to eliminate interference
[0088]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com