Production process of organosilane polysulfide
A production process, organosilane technology, applied in the field of organosilane polysulfide production process, can solve the problems of poor storage stability of finished products, high content, difficult control of process conditions, etc., and achieve the effect of uniform and stable color and small dosage of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
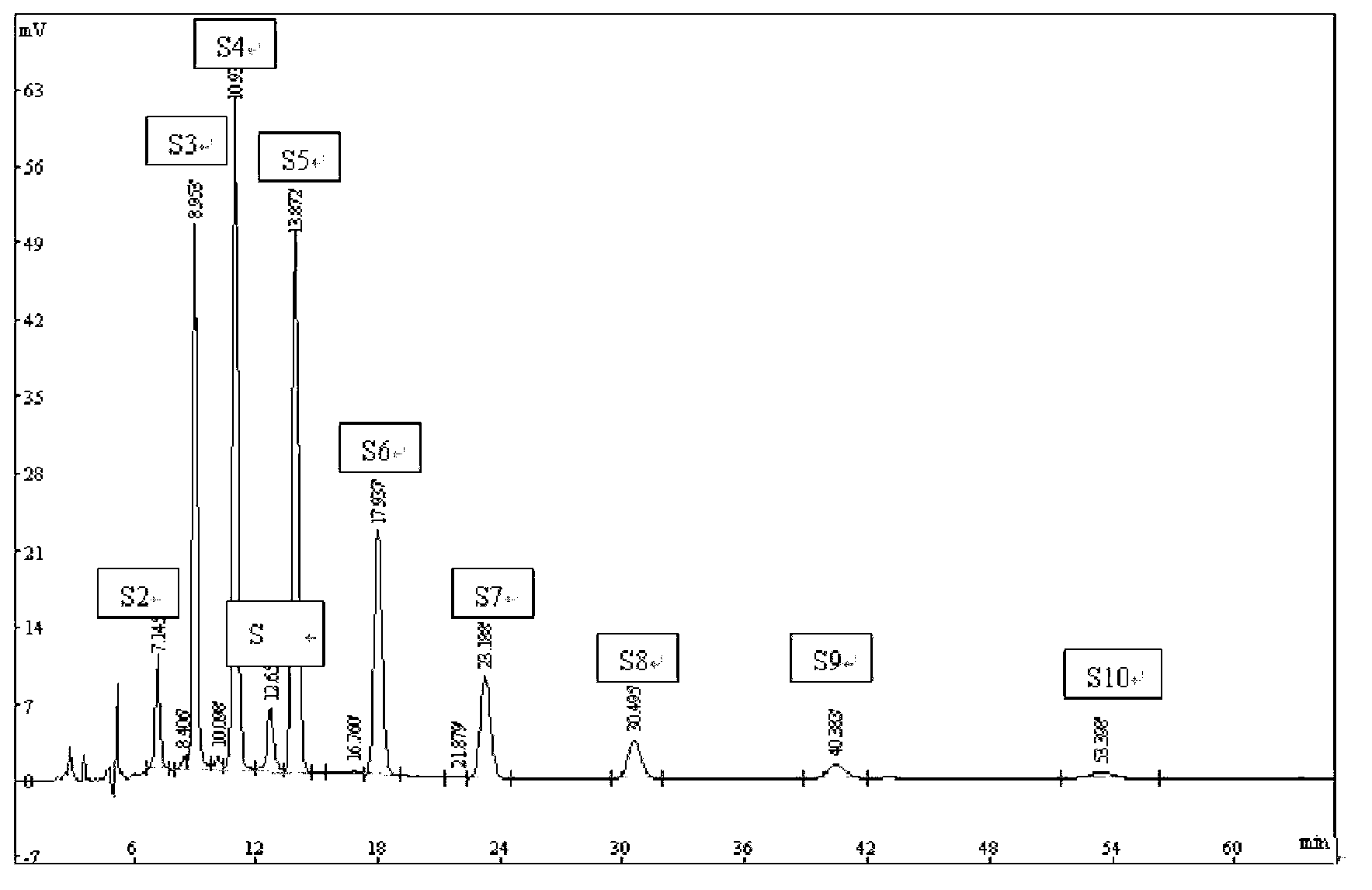
Image
Examples
Embodiment 1
[0056] (1) Put 24 kg of sodium hydroxide aqueous solution into the sulfur-melting kettle, heat it to 90-100°C, then put 16 kg of sulfur into the sulfur-melting kettle, control the reaction temperature at 90-100°C, keep it warm for 1 hour, and then Throw in 16kg of sulfur and keep warm for 1 to 3 hours. The resulting sodium polysulfide reaction solution was directly used in the next step without separation.
[0057] (2) Pump the sodium polysulfide reaction solution obtained in step (1) into the condensation reaction kettle, control the temperature at 80-82°C, add 4 kg of tetrabutylammonium bromide aqueous solution as a phase transfer catalyst, and add 5% bicarbonate Sodium solution is used as a hydrolysis buffer to make the pH value 9-10. After 5 minutes, add 96 kg γ-chloropropyltriethoxysilane (γ2) for condensation reaction, the reaction temperature is 85±2°C, and the time is 60 minutes. Stop the reaction after sampling and testing the residual γ2 content of 0.8-1.5%.
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com