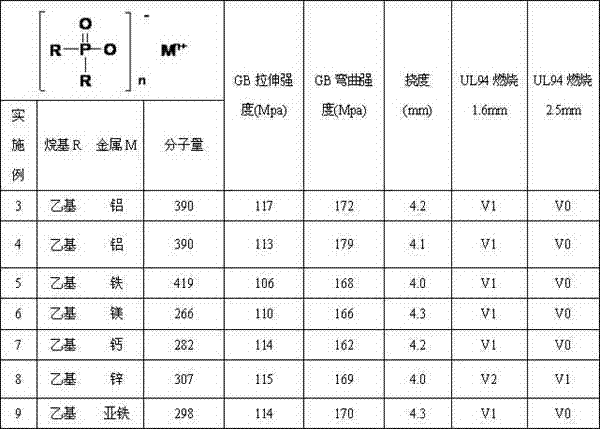
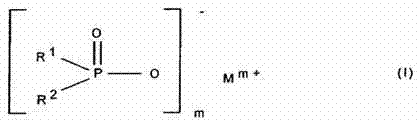
Dialkyl phosphinic acid salt and preparation method thereof
A technology of dialkyl phosphinate and alkyl phosphinate is applied in the field of dialkyl phosphinate and its preparation, and can solve the problems of easy quenching and deactivation of free radical initiators, long reaction period and the like, Achieve the effect of high yield, short reaction period and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of high concentration hypophosphorous acid
[0056] Get 400.1g mass fraction to be 50% hypophosphorous acid and 250ml cyclohexane, add in the reaction bottle that oil-water separator and condenser are housed, fill up cyclohexane in oil-water separator;
[0057] Heat and stir under nitrogen protection until azeotropic for about 3 hours, and discharge the water separated at the bottom of the oil-water separator in stages; add the solution in the reaction bottle to a separatory funnel to separate to obtain 222.2 g of the lower liquid, and use acid-base titration to obtain the acid content For: 90.38% (mass fraction).
Embodiment 2
[0058] Example 2: Preparation of high concentration hypophosphorous acid
[0059] Get 400.1g mass fraction 50% hypophosphorous acid and 250ml cyclohexane, add in the reaction bottle that oil-water separator and condenser are housed, fill up cyclohexane in oil-water separator;
[0060] Heat and stir under nitrogen protection until azeotropic for about 4 hours, discharge the water separated at the bottom of the oil-water separator in stages; add the solution in the reaction bottle to a separatory funnel to separate to obtain 212.4 g of the lower layer liquid, and use acid-base titration to obtain the acid content For: 94.07% (mass fraction).
[0061] Get 200.2g of hypophosphorous acid and 200ml methyl alcohol of 94.07% mass fraction, add in the reaction flask that oil-water separator and condenser are housed, and fixed solid calcium chloride is housed in oil-water separator and condenser wherein and in oil-water separator Fill with methanol, heat and stir to azeotrope for 4...
Embodiment 3
[0063] Prepare the high-concentration hypophosphorous acid of 99.49% mass fraction with embodiment 2, get the high-concentration hypophosphorous acid of 99.49% mass fraction
[0064] Add 181.2g (2.732mol), 150ml of benzene and 2.4g (0.014mol, 0.5%mol) of tert-butyl peroxypivalate into the autoclave, seal the autoclave, and replace it with nitrogen (0.5MPa) for 5 times while stirring , and then filled with ethylene to 2.5MPa saturation, heated to 70°C under stirring, and added 4.7g (0.027mol, 1.0%mol) of tert-butyl peroxypivalate and 50ml of benzene evenly within 4 hours. , uniformly warming up to 80° C. within 5 hours, cooling the reactor and venting to obtain 468.8 g of diethylphosphinic acid homogeneous solution containing telomer, acid content: 71.02% (mass fraction);
[0065] Diethylphosphinic acid homogeneous solution 468.8g, extract three times with 300ml water, obtain diethylphosphinic acid aqueous solution altogether
[0066] 1192.3g, the recorded acid content is 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com