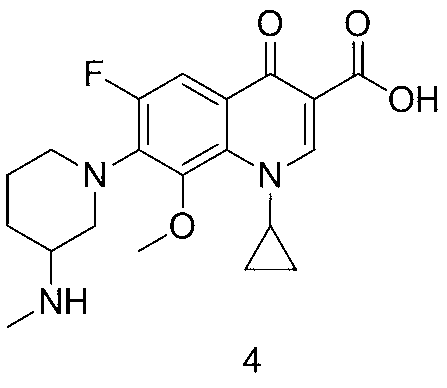
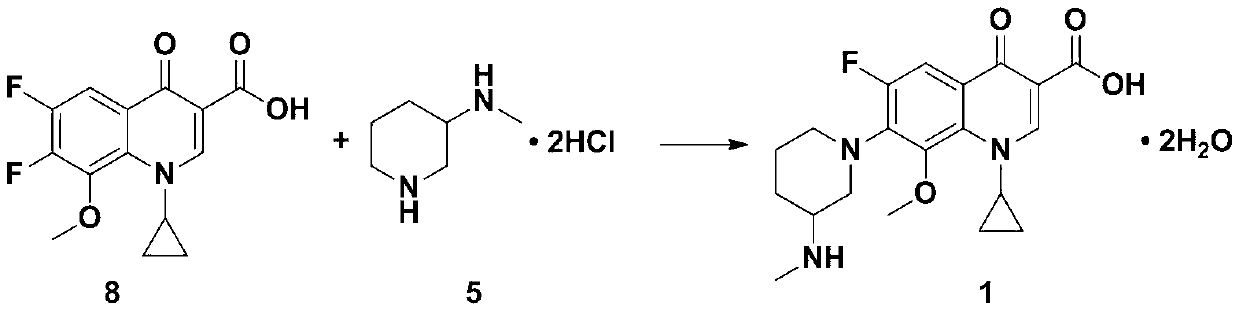
Preparation method of balofloxacin dihydrate
A technology for baloxacin dihydrate and compounds, which is applied in the field of preparation of baloxacin dihydrate, can solve the problems of poor stability of acetyl boric acid chelates, etc., and achieve simple operation, high total yield, and improved The effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
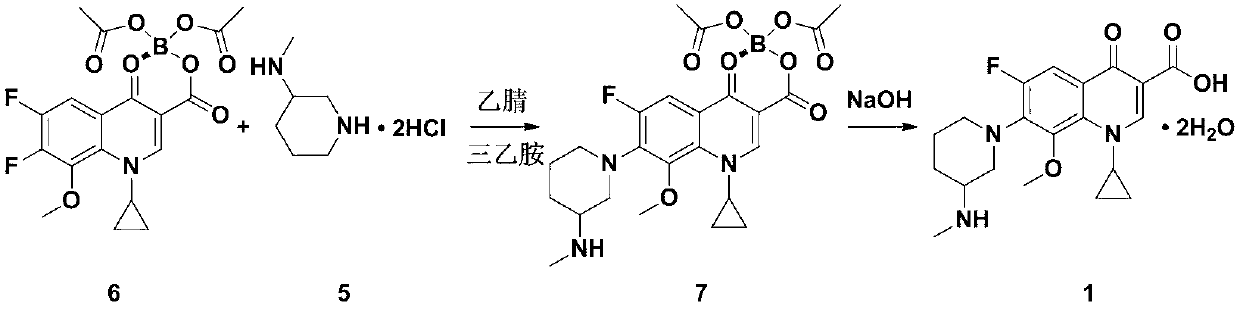
[0039] The synthesis circuit of the present embodiment is basically:
[0040]
[0041] The compound of formula 2 (15g, 33.26mmol) was dissolved in 60mL of acetonitrile, triethylamine (18mL, 129.14mmol) and 3-methylaminopiperidine dihydrochloride (6.5g, 34.74mmol) were added once at room temperature, and the temperature was kept at 30 ~35°C. After reacting for 12 hours, acetonitrile was recovered by distillation under reduced pressure to obtain 28.9 g of crude yellow compound. The crude yellow compound was directly used in the next reaction. The yellow compound analysis sample was purified by column chromatography and detected as the compound of formula 3. ESI-MS m / z: 546[M+H] +1 . H-NMR (CDCl 3 )δ: 1.02(6H, t), 1.3-1.26(2H, t), 1.34-1.43(2H, m), 1.75-1.85(1H, m), 1.90-2.04(2H, m), 2.35-2.41( 4H, m), 2.43-2.53(1H, m), 2.73(3H, s), 3.26-3.32(2H, m), 3.53-3.59(2H, t), 3.77(3H, s), 3.97-3.99( 1H, d) 4.21-4.26(1H, m), 7.97(1H, d), 9.14(1H, s).
[0042] Add 9 g of sodium ...
Embodiment 2-6
[0045] Using the same reaction conditions as in Example 1, 5 batches of baloxacin dihydrate were prepared.
[0046] Table 2 Compound of Formula 2 Preparation of Compound of Formula 1 through Intermediate Compound of Formula 3 Results List
[0047]
[0048] Note: The dosage of the compound of formula 5 is 6.7g, the dosage of triethylamine is 18mL, and the dosage of acetonitrile is 60mL.
Embodiment 7
[0051] The compound of formula 2 (15g, 33.26mmol) was dissolved in 60mL DMF, triethylamine (18mL, 129.14mmol) and 3-methylaminopiperidine dihydrochloride (6.5g, 34.74mmol) were added once at room temperature, and the temperature was kept 30-35°C, reacted for 12 hours, and recovered DMF by distillation under reduced pressure to obtain 35.5 g of crude yellow compound, which was directly used in the next reaction.
[0052] Add 100mL aqueous solution of 9g sodium hydroxide to the crude compound of formula 3, slowly raise the temperature to 65-70°C and react for 2.5h, the reaction solution is cooled to room temperature, filtered, the filtrate is adjusted to pH 7.5 with 4M hydrochloric acid, stirred at room temperature for 1h, cooled to 4°C, let stand overnight, and filter. Add 100mL of 7% acetic acid to the obtained solid, stir at room temperature for 2h, filter, adjust the pH of the filtrate to 7.5 with 10% aqueous sodium hydroxide solution, stir at room temperature for 2h, cool t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com