Baby bifidobacterium and preparation thereof
A technology for Bifidobacterium infantis and preparations, which is applied to bacteria, medical raw materials derived from bacteria, medical preparations with inactive ingredients, etc. requirements, etc., to achieve the effect of improving the viable bacteria rate and its storage time, quality and production conditions, and enhancing the activity of seeds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The present invention also provides a kind of preparation method of freeze-dried powder preparation of the present invention, described method comprises the following steps:
[0068] (a) Inoculate and cultivate the Bifidobacterium infantis bacterial classification of the present invention in a primary seed liquid culture medium to obtain a primary inoculation product;
[0069] (b) inoculating and culturing the primary inoculation product into the secondary seed liquid medium to obtain the secondary inoculation product;
[0070] (c) the secondary inoculation product is connected to the culture medium in the 50L fermenter to obtain the fermentation product;
[0071] (d) connecting the fermented product obtained in step (c) to the culture medium of the fermenter to obtain the fermented product;
[0072] (e) centrifuging the fermentation product obtained in step (d), mixing it with the protective agent, and freeze-drying to obtain a freeze-dried powder preparation.
[007...
Embodiment 1
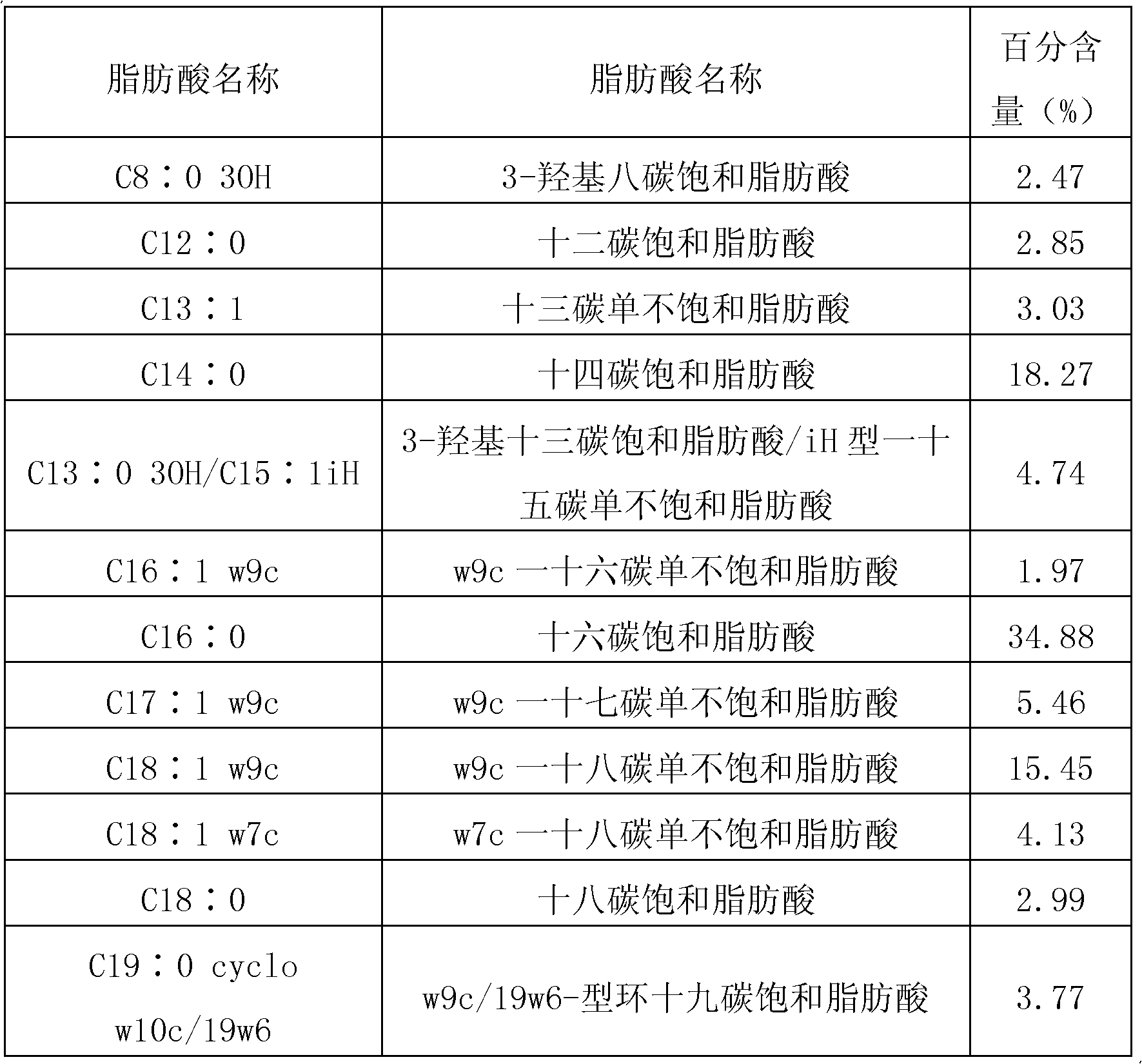
[0082] Embodiment 1. prepare the bacterial classification of bifidobacterium infantis
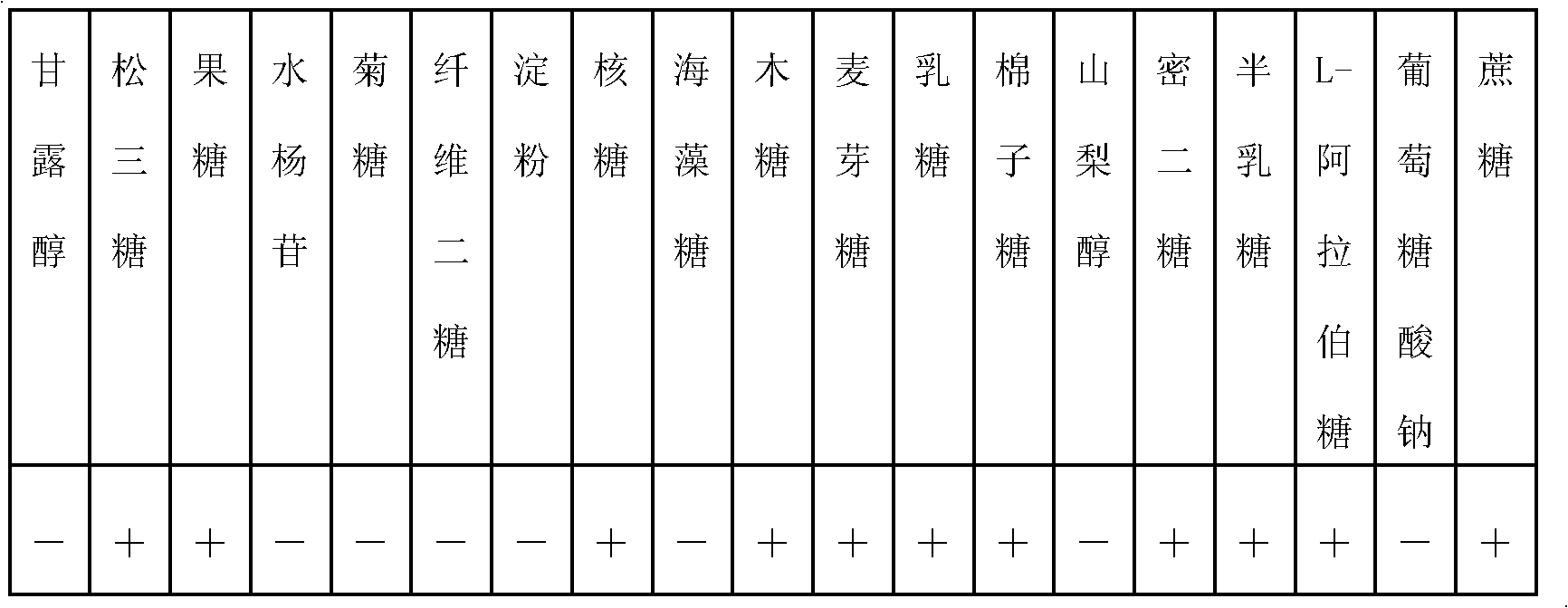
[0083] A. Prepare PYG liquid medium (proportion 1). Use 50mL pure water to dissolve tryptone (MERCK KGAA, Germany), yeast extract (OXID, UK), L-cysteine hydrochloride (Shanghai Kangjie Biotechnology Co., Ltd.), glucose (Shanxi Wang Biochemical Technology Co., Ltd.), calcium chloride (Sinopharm Chemical Reagent Co., Ltd.), magnesium sulfate (Sinopharm Chemical Reagent Co., Ltd.), dipotassium phosphate (Sinopharm Chemical Reagent Co., Ltd.), potassium dihydrogen phosphate (Sinopharm Chemical Reagent Co., Ltd.) to obtain a suspension, and adjust the pH of the medium with a little sodium hydroxide solution. Put the culture medium in the Erlenmeyer flask, seal the mouth of the flask and place it in a sterilizer for sterilization at 121°C for 15 minutes. Add fresh feces of healthy babies (taken from China Welfare Institute International Peace Maternal and Child Health Hospital) to the PYG liq...
Embodiment 2
[0091] Embodiment 2. prepare the primary seed liquid culture medium of Bifidobacterium infantis
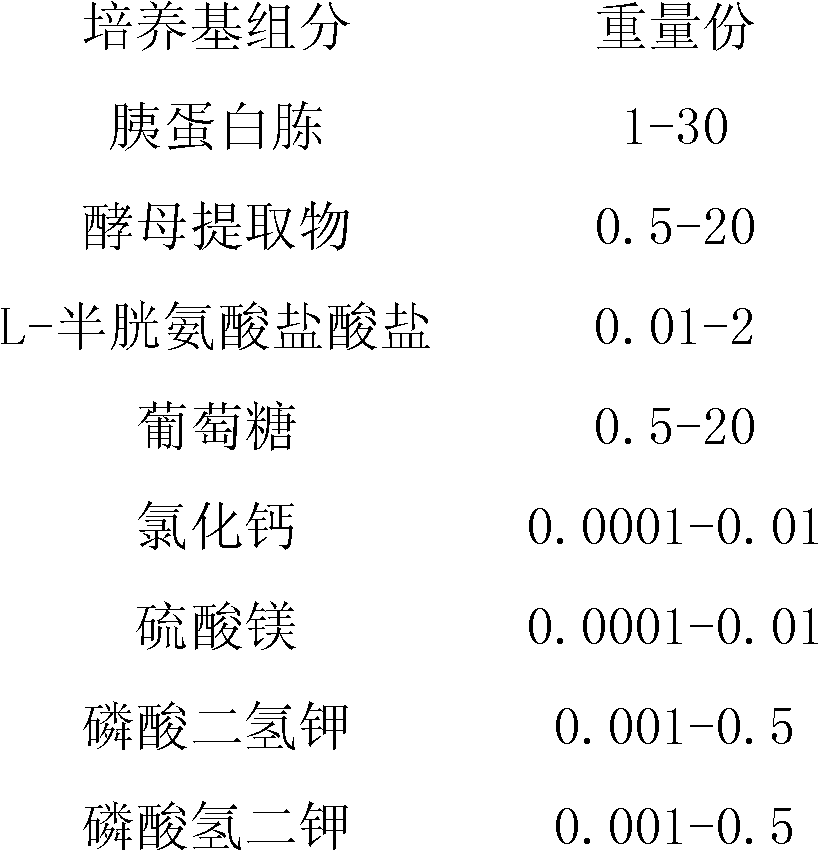
[0092] Use 300 mL of pure water to dissolve tryptone (MERCK KGAA, Germany), beef extract (OXID, UK), yeast extract (OXID, UK), lactose (DMV-Fonterra Excipients, Germany), glucose (Shandong Xiwang Biochemical Technology Co., Ltd. Co., Ltd.), dipotassium hydrogen phosphate (Sinopharm Group Chemical Reagent Co., Ltd.), magnesium sulfate (Sinopharm Group Chemical Reagent Co., Ltd.), ferrous sulfate (Sinopharm Group Chemical Reagent Co., Ltd.), L-cysteine hydrochloride ( Shanghai Kangjie Biotechnology Co., Ltd.) to obtain a suspension, and adjust the pH of the medium with a little sodium hydroxide solution. Put the culture medium in the Erlenmeyer flask, seal the mouth of the flask and place it in a sterilizer for sterilization at 121°C for 15 minutes.
[0093] Table 6. Ratio of primary seed liquid culture medium
[0094]
[0095]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com