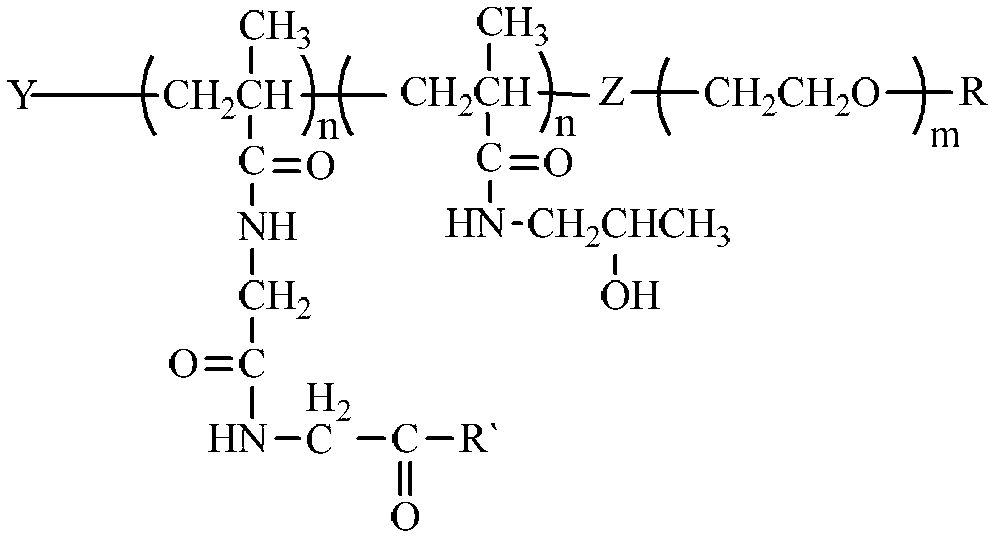Long-circulating anti-tumor targeting drug carrier and preparation method thereof
A monomer and polymer technology, applied in the field of long-circulating anti-tumor targeted drug carrier and its preparation, can solve the problems of non-specific protein adsorption, wide molecular weight distribution, uncontrollable molecular weight, etc. The effect of adsorption, narrow molecular weight distribution and controllable molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
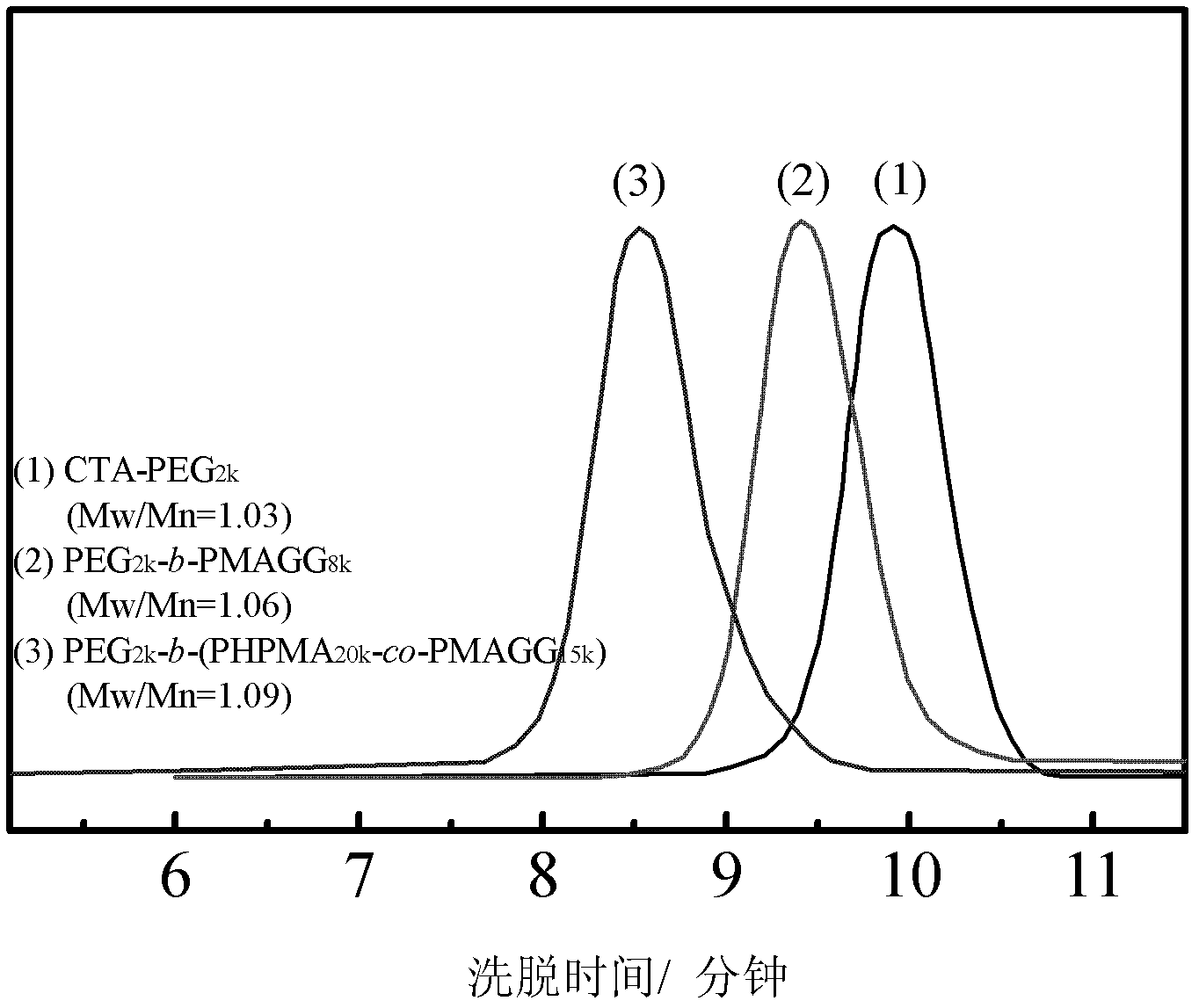
[0033] Embodiment 1, PEG 2k -b-PMAGG 8k preparation of
[0034] Dissolve 2.4 g of hydroxyl- and methyl-terminated PEG with a number average molecular weight of 2000 in 40 mL of toluene, add 0.68 g of 4-dithiobenzoate-4-cyanovaleric acid (CPAD) and 0.041 g of DMAP, and wait After complete dissolution, 0.74 g of DCC was added and reacted for 90 hours under stirring at room temperature. Suction filtration, pour the filtrate into excess diethyl ether, suction filtration, the resulting precipitate was dissolved in a small amount of toluene, and then precipitated with diethyl ether, and this was repeated three times. The precipitate was vacuum-dried at 40° C. for 24 hours, and the obtained product was a macromolecular chain transfer agent of PEG.
[0035] Take 0.13g of this chain transfer agent, 0.46g of MAGG, 5mg of 4,4'-azobis(4-cyanovaleric acid) and 5mL of pure water, put them in a vacuum reaction tube, vacuumize under refrigeration, and then return to room temperature, Infl...
Embodiment 2
[0038] Example 2, PEG 2k -b-(PHPMA 20k -co-PMAGG 15k ) preparation
[0039] The preparation method of the block copolymer is basically the same as in Example 1, except that the original MAGG is changed to a mixture of HPMA and MAGG for feeding, and the feeding amounts are 1.16 g of HPMA and 0.87 g of MAGG. When the reaction was terminated, the reaction tube was quickly put into liquid nitrogen, and then the small molecules were removed by dialysis, and finally the solvent was evaporated to obtain the desired product. The molecular weight and composition of the product can be determined by aqueous GPC and 1 HNMR to co-confirm.
[0040] Products with different compositions and molecular weights can be obtained according to different reaction times. If the reaction time is 48 hours, the number average molecular weight of the obtained copolymer is 37000, the molecular weight distribution is 1.09, the molecular weight of the PMAGG part is 15000, and the molecular weight of the...
Embodiment 3
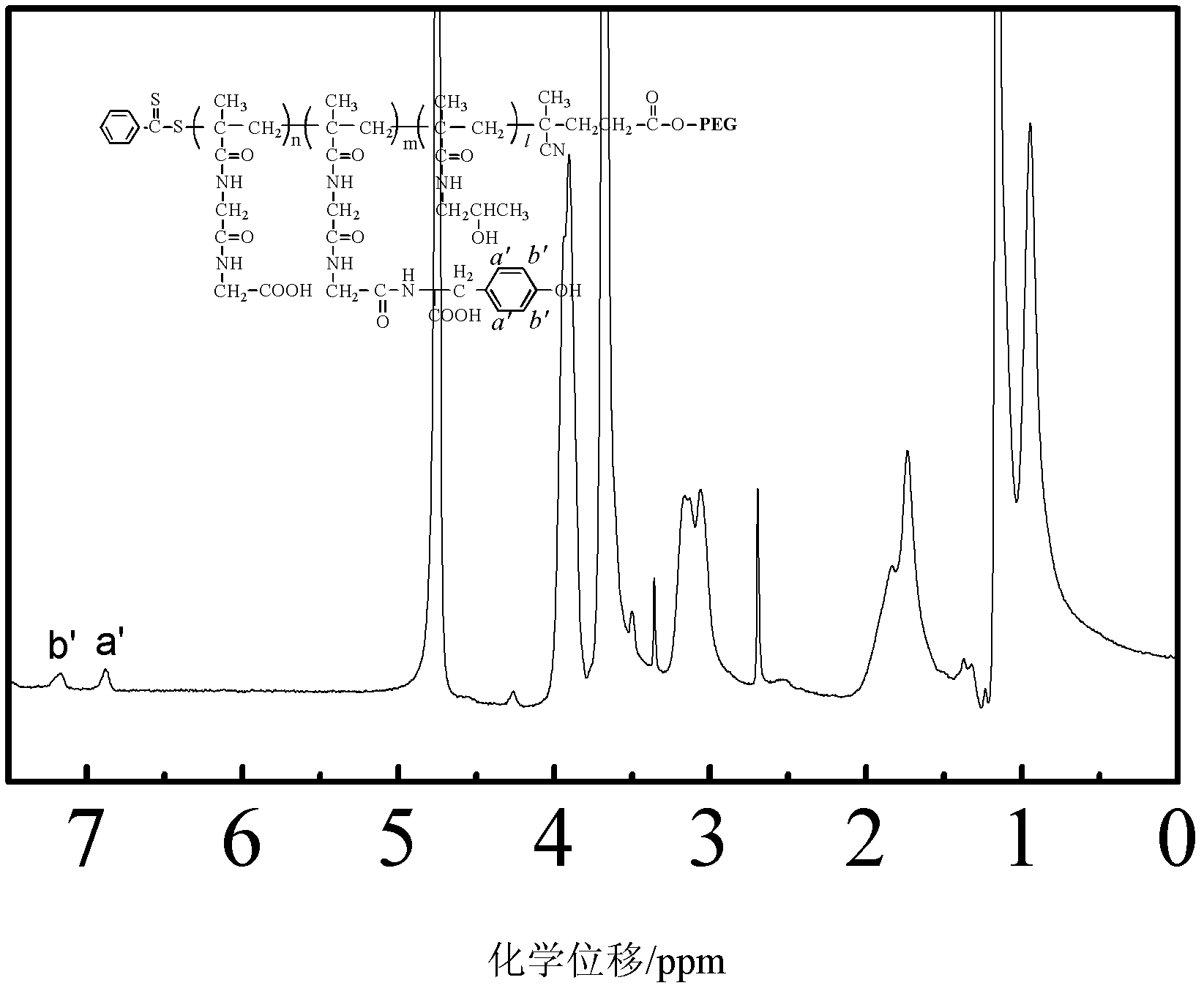
[0041] Embodiment 3, polymer PEG containing amino acid 2k -b-PMAGG 20k preparation of
[0042] 2.2g polymer PEG 2k -b-PMAGG 20k With 0.072g tyrosine in 10ml mass concentration of 10% sodium carbonate aqueous solution, with 0.191g EDC and 0.115g (NHS) as a catalyst, react overnight at 4 ° C, then dialyze in pure water for 4 × 6h, freeze-dried Polymer PEG containing tyrosine 2k -b-PMAGG 20k . That 1 H NMR spectrum see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com