Heparin sodium tube sealing injection and preparation method thereof
A tube-locking injection and heparin sodium technology, which is applied in the field of heparin sodium tube-locking injection and its preparation, can solve the problems of interference of the test results of the injection, poor stability of the injection, and inability to use children, etc., so as to avoid local Effects of blood coagulation and blockage, reduction of local irritation and bleeding, and improvement of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
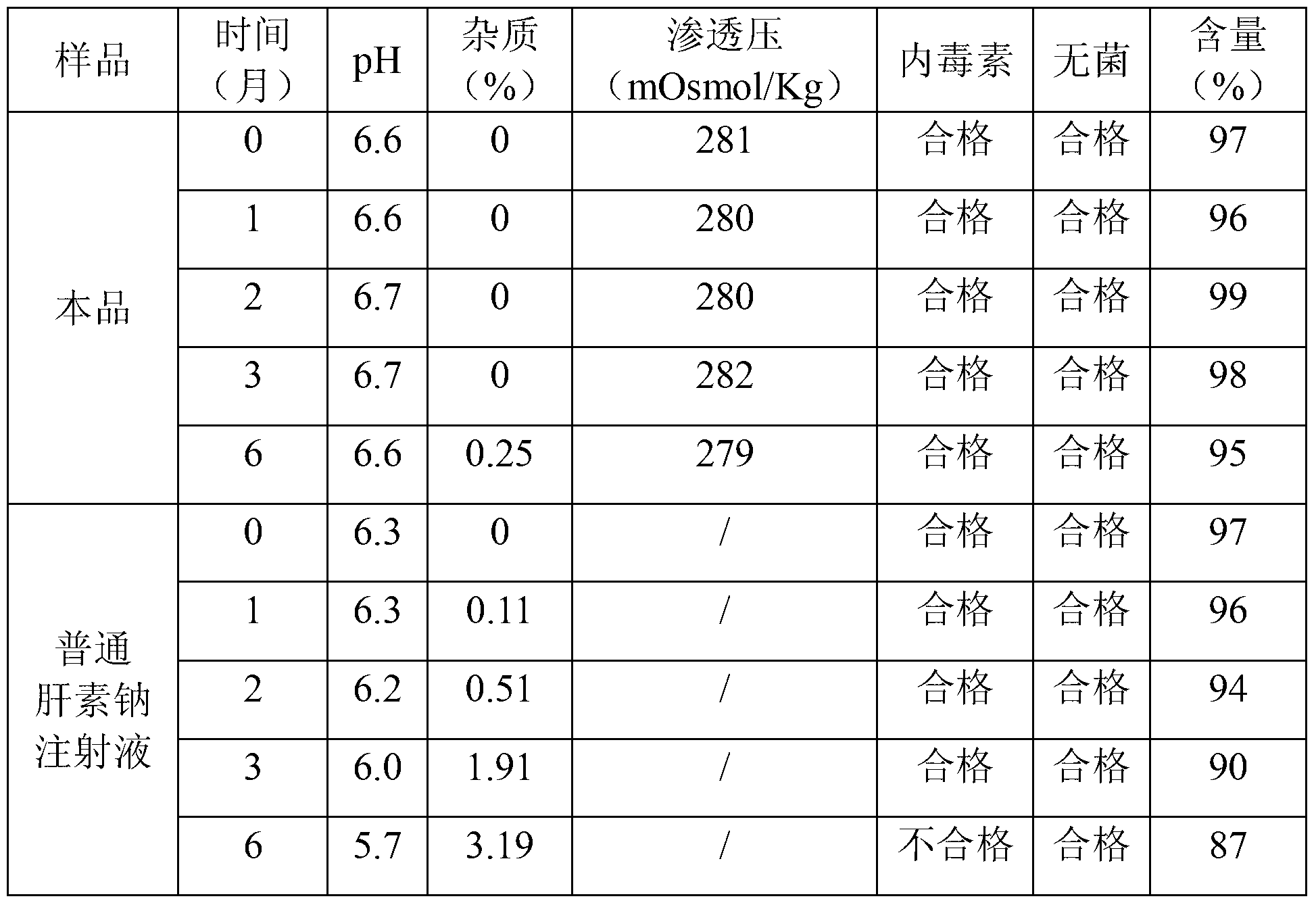
[0025] Take 5 million units of heparin sodium, 1,250,000 mg of glucose, 50,000 mg of mannitol, 200,000 mg of sodium dihydrogen phosphate, and 300,000 mg of disodium hydrogen phosphate, dissolve them in an appropriate amount of water for injection, measure the pH value, and adjust it to 6 with a pH regulator such as NaOH or phosphoric acid if necessary. Between ~7, add water to 50000ml, filter with 0.1 micron microporous membrane, fill in ampoules, each 5ml or 10ml, seal. High-temperature sterilization at 115°C for 30 minutes, lamp inspection, and packaging to obtain 5ml: 500 units sealed tube injection. The accelerated test (40°C±2°C, RH=75%±5%) of this product and ordinary heparin sodium tube locking injection was compared, and the results showed that this product has better efficacy than ordinary heparin sodium locking injection stability. The specific test conditions are shown in the table below:
[0026] Stability comparison test results (1)
[0027]
Embodiment 2
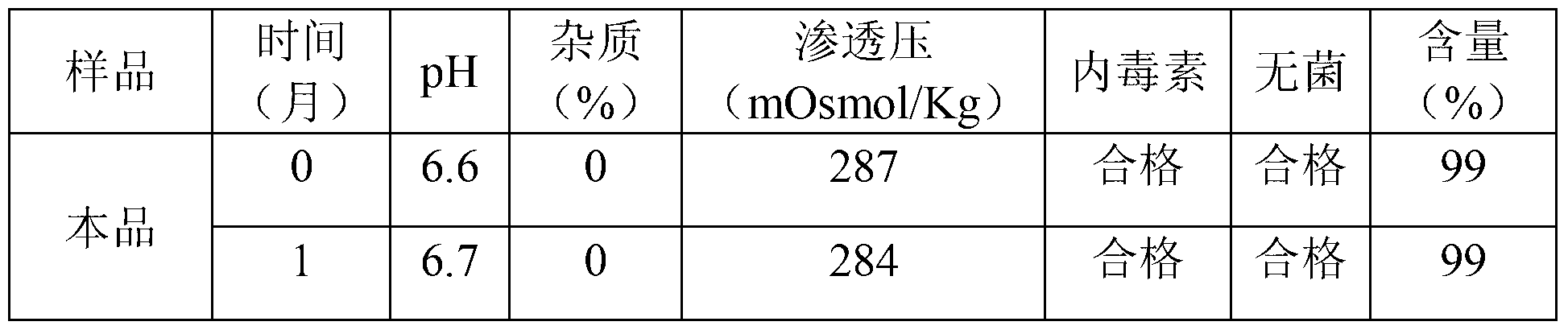
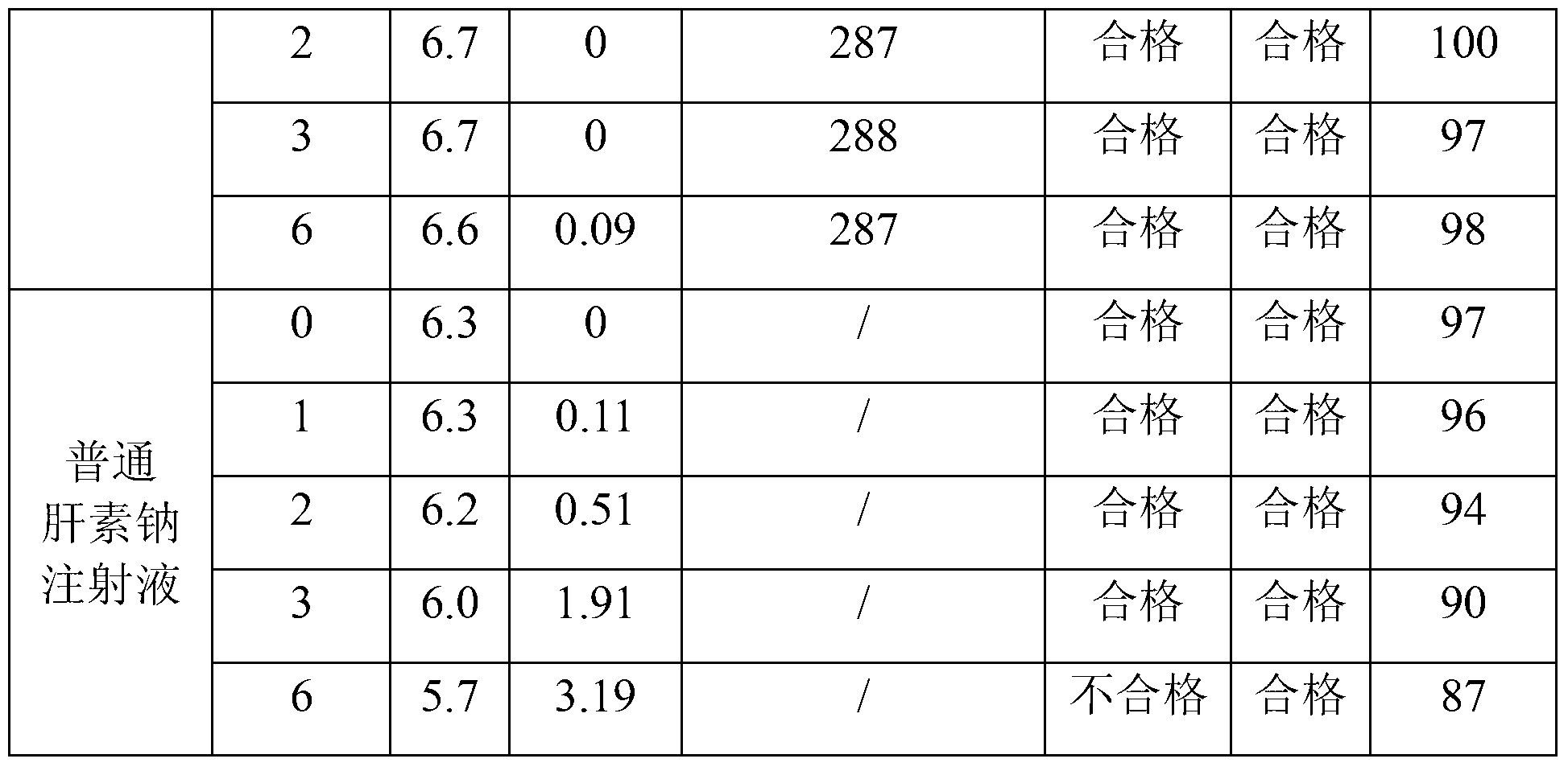
[0029]Take 500,000 units of heparin sodium, 1,250,000 mg of glucose, 50,000 mg of mannitol, 200,000 mg of sodium dihydrogen phosphate, and 300,000 mg of disodium hydrogen phosphate, dissolve them in an appropriate amount of water for injection, measure the pH value, and adjust it to 6 with a pH regulator such as NaOH or phosphoric acid if necessary. Between ~7, add water to 50000ml, filter with a 0.05 micron microporous membrane, fill in ampoules, 5ml or 10ml each, and seal. High-temperature sterilization at 115°C for 30 minutes, light inspection, and packaging to obtain 5ml: 50 units sealed tube injection. The accelerated test (40°C±2°C, RH=75%±5%) of this product and ordinary heparin sodium tube locking injection was compared, and the results showed that this product has better efficacy than ordinary heparin sodium locking injection stability. The specific test conditions are shown in the table below:
[0030] Stability comparison test results (2)
[0031]
[0032] ...
Embodiment 3
[0034] Take 5 million units of heparin sodium, 1,250,000 mg of glucose, 50,000 mg of mannitol, 200,000 mg of sodium dihydrogen phosphate, and 300,000 mg of disodium hydrogen phosphate. In order to further reduce the pain of injection, 50,000 mg of lidocaine hydrochloride was dissolved in an appropriate amount of water for injection, and the pH value was measured. , if necessary, use NaOH or phosphoric acid and other pH regulators to adjust to between 6 and 7, add water to 50,000ml, filter with a 0.2 micron microporous membrane, fill in ampoules, 5ml or 10ml each, and seal. High-temperature sterilization at 115°C for 30 minutes, lamp inspection, and packaging to obtain 5ml: 500 units sealed tube injection. The accelerated test (40°C±2°C, RH=75%±5%) of this product and ordinary heparin sodium tube locking injection was compared, and the results showed that this product has better efficacy than ordinary heparin sodium locking injection stability. The specific test conditions are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com