Preparation method of stable chitosan nano-micelle with CO2 responsiveness and temperature responsiveness
A temperature-responsive, nanomicelle technology, applied in the fields of polymer materials and biomedical engineering, can solve the problems of poor solubility and single function, and achieve the effect of simple and easy synthesis method and wide source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
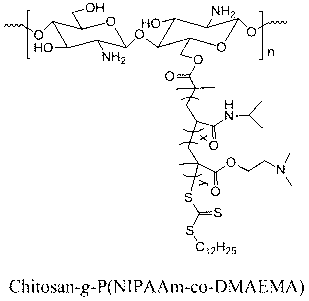
[0021] Weigh 2.0 grams of chitosan in a reaction kettle, disperse and dissolve it with N,N-dimethylformamide, and then add 15 grams of N,N-dicyclohexylcarbodiimide. Dissolve 16 grams of 2-(dodecyltrithiocarbonate)-2-methylpropionic acid in N,N-dimethylformamide and drop it into the reaction kettle at 0°C for 20 minutes After dropping, react at 10°C for 72 hours. After desalination by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is chitosan is obtained. Weigh 0.2 g of RAFT macromolecular chain transfer agent and dissolve it in dioxane, add 5.2 g of N-isopropylacrylamide, 0.8 g of N,N-dimethylaminoethyl methacrylate, and then add the initiator azo 0.002 g of diisobutyronitrile was vacuum-filled three times with nitrogen, and reacted in an oil bath at 20° C. for 48 hours under nitrogen protection. The product was dialyzed against deionized water and freeze-dried to obtain CO with chitosa...
Embodiment 2
[0023] Weigh 2.0 grams of chitosan, disperse and dissolve it with N,N-dimethylformamide, add 10 grams of chlorosulfonic acid, and dissolve 20 grams of 2-(dodecyltrithiocarbonate)-2-methyl Propionic acid was dissolved in N,N-dimethylformamide, dropped into the reaction kettle at 5°C, dropped in 30 minutes, and reacted at 15°C for 54 hours. After desalination by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is chitosan is obtained. Weigh 0.2 g of RAFT macromolecular chain transfer agent and dissolve it in N,N-dimethylacetamide, add 4.9 g of N-isopropylacrylamide, 1.1 g of N,N-dimethylaminoethyl methacrylate, and then Add 0.003 g of azobisisobutyronitrile as an initiator, go through the process of evacuating and filling with nitrogen three times, and react in an oil bath at 40° C. for 36 hours under the protection of nitrogen. The product was dialyzed against deionized water and freeze-dri...
Embodiment 3
[0025] Weigh 2.0 grams of chitosan, disperse and dissolve it with N,N-dimethylacetamide, add 15 grams of thionyl chloride, and dissolve 25 grams of 2-(dodecyltrithiocarbonate)-2- Methylpropionic acid was dissolved in N,N-dimethylacetamide, dropped into the reaction kettle at 10°C for 40 minutes, and reacted at 20°C for 36 hours. After desalination by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is chitosan is obtained. Weigh 0.2 g of RAFT macromolecular chain transfer agent and dissolve it in N,N-diethylformamide, add 4.7 g of N-isopropylacrylamide, 1.3 g of N,N-dimethylaminoethyl methacrylate, and then Add 0.004 g of azobisisobutyronitrile as an initiator, go through the process of evacuating and filling nitrogen three times, and react in an oil bath at 50° C. for 10 hours under the protection of nitrogen. The product was dialyzed against deionized water and freeze-dried to obtain CO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com