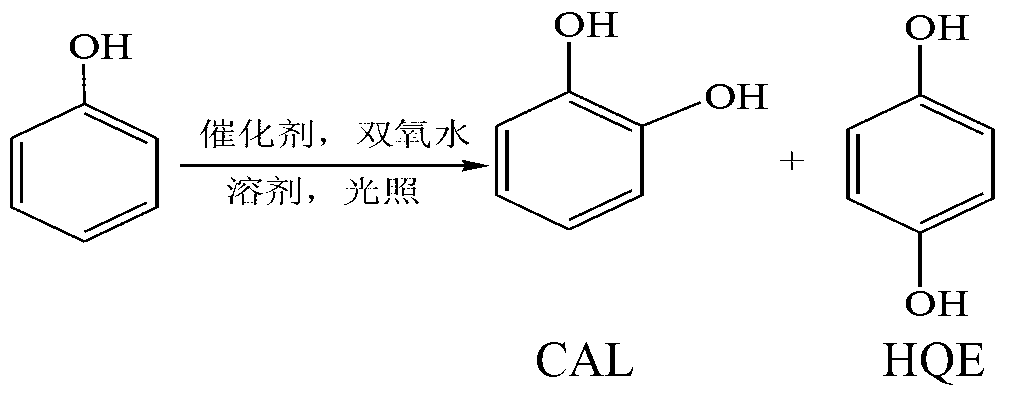
Photocatalyst for synthesizing o-(p-)benzenediol, and synthetic method for o-(p-)benzenediol
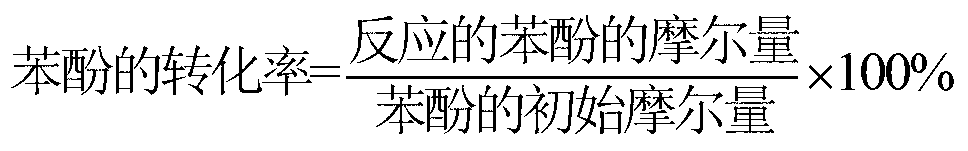
A technology of photocatalyst and hydroquinone, applied in the field of photocatalytic reaction, can solve the problems of low selectivity of CAL and HQE, low yield of CAL and HQE, low conversion rate of phenol, etc., achieve high yield and great industrial application value , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The material ratio is:
[0039] TiO 2 : C.I. Pigment Yellow 154=1:0.09 (mass ratio);
[0040] TiO 2 : dimethyl sulfoxide = 1: 16 (mass ratio);
[0041] TiO 2 : Tween-80 = 1:0.12 (mass ratio);
[0042] Add 0.9 g of C.I. Pigment Yellow 154 to the beaker, and then add 160 g of dimethyl sulfoxide, and stir to dissolve it. Then add 10g TiO to it 2 (commercial brand P25), and add 1.2g Tween-80, stir until a yellow uniform suspension slurry is formed. The suspension slurry system was transferred to a stainless steel autoclave, and treated at a constant temperature of 190° C. for 4 hours. After the treatment is completed, it is naturally cooled to room temperature. The obtained suspension slurry was separated by filtration, and the obtained filter cake was washed with distilled water several times until the filtrate was colorless. Then place the obtained precipitate in a drying oven to dry at 100°C, and grind it into powder after drying completely to obtain light yello...
Embodiment 2
[0044] The material ratio is:
[0045] TiO 2 : C.I. Pigment Yellow 154=1:0.05 (mass ratio);
[0046] TiO 2 : dimethyl sulfoxide = 1: 13 (mass ratio);
[0047] TiO 2 : Tween-80 = 1:0.05 (mass ratio);
[0048] Add 0.5 g of C.I. Pigment Yellow 154 to the beaker, and then add 130 g of dimethyl sulfoxide, and stir to dissolve it. Then add 10g TiO to it 2(commercial brand P25), and add 0.5g Tween-80, stir until a yellow uniform suspension slurry is formed. The suspension slurry system was transferred to a stainless steel autoclave, and treated at a constant temperature of 210° C. for 3 hours. After the treatment is completed, it is naturally cooled to room temperature. The obtained suspension slurry was separated by filtration, and the obtained filter cake was washed with distilled water several times until the filtrate was colorless. Then place the obtained precipitate in a drying oven to dry at 100°C, and grind it into powder after drying completely to obtain light yellow...
Embodiment 3
[0050] The material ratio is:
[0051] TiO 2 : C.I. Pigment Yellow 154=1:0.1 (mass ratio);
[0052] TiO 2 : dimethyl sulfoxide = 1:20 (mass ratio);
[0053] TiO 2 : Tween-80 = 1:0.15 (mass ratio);
[0054] Add 1 g of C.I. Pigment Yellow 154 to the beaker, and then add 200 g of dimethyl sulfoxide, and stir to dissolve it. Then add 10g TiO to it 2 (commercial brand P25), and add 1.5g Tween-80, stir until a yellow homogeneous suspension slurry is formed. The suspension slurry system was transferred to a stainless steel autoclave, and treated at a constant temperature of 180° C. for 2 hours. After the treatment is completed, it is naturally cooled to room temperature. The obtained suspension slurry was separated by filtration, and the obtained filter cake was washed with distilled water several times until the filtrate was colorless. Then place the obtained precipitate in a drying oven to dry at 100°C, and grind it into powder after drying completely to obtain light yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com