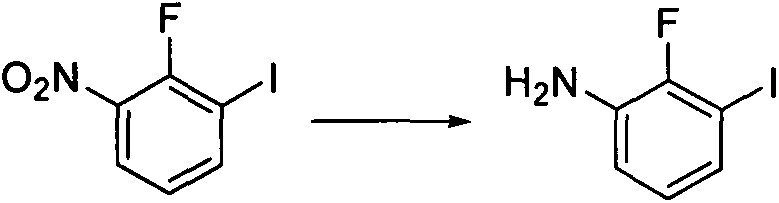
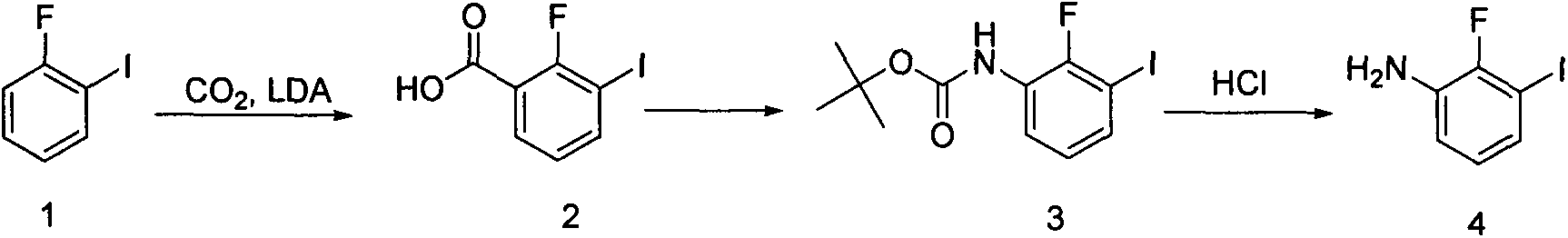Preparation method of 2-fluoro-3-iodoaniline
A technology of iodoaniline and fluoroiodine, which is applied in the field of preparation of 2-fluoro-3-iodoaniline, can solve the problems of complex products, difficulty in controlling reaction conditions, and many by-products of iodine removal, so as to achieve cheap and easy-to-obtain raw materials and avoid product Decomposition, the effect of improving yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
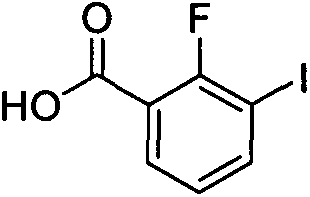
[0018] Embodiment 1: Synthesis of 2-fluoro-3-iodobenzoic acid (compound 2)
[0019]
[0020] Diisopropylamine (13 ml, 91.0 mmol) was dissolved in tetrahydrofuran (100 ml), and 2.5M n-butyllithium solution (36.4 ml, 91.0 mmol) was added dropwise at -20°C under nitrogen protection. After dropping, stir at 0°C for 30 minutes. Then, a solution of o-fluoroiodobenzene (18.4 g, 82.8 mmol) in tetrahydrofuran (50 mL) was added dropwise, and stirring was continued at -70°C for 1 hour. Dry ice (50 g) was added, allowed to warm to room temperature and stirred overnight. 100 ml of water was added to the above reaction mixture, and the organic phase was separated. The aqueous phase was acidified with concentrated hydrochloric acid to pH 1-2, extracted three times with ethyl acetate, and dried over anhydrous sodium sulfate. Concentration under reduced pressure afforded 15.5 g of yellow solid (2), yield: 70.5%.
Embodiment 2
[0021] Example 2: Synthesis of tert-butyl 2-fluoro-3-iodophenylcarbamate (compound 3)
[0022]
[0023] Compound (2) (13.3g, 50.0mmol), triethylamine (20.8ml, 150.0mmol) and DPPA (26.7g, 100.0mmol) were dissolved in tert-butanol (50ml) and toluene (50ml), Heat to 80°C and stir overnight. Distilled under reduced pressure, the residue was subjected to silica gel column chromatography (200-300 mesh, petroleum ether: ethyl acetate = 100:1-20:1), and vacuum-dried to obtain 9.31 g of colorless liquid (3), yield: 55.3% .
Embodiment 3
[0024] Embodiment 3: Synthesis of 2-fluoro-3-iodoaniline (compound 4)
[0025]
[0026] Compound (3) (9.31 g, 27.7 mmol) was dissolved in methanol (50 mL), concentrated hydrochloric acid (10 mL) was added, and stirred at room temperature for 3 hours. The methanol was distilled off under reduced pressure, and the saturated sodium bicarbonate solution was basified to pH 8-9. Extracted with ethyl acetate, dried over anhydrous sodium sulfate. Concentration under reduced pressure afforded 6.6 g of off-white solid (4), yield: 100%.
[0027] 1 H-NMR (DMSO-d 6 , 400MHz): δ6.35~6.49 (2H, m), δ7.01 (1H, d), δ4.19 (2H, brs).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com