Nitrogen-containing bidentate heterocyclic substituted tetrazole rare earth complex and preparation method thereof
A technology for rare earth complexes and azole compounds is applied in the field of synthesis of tetrazole ligands and rare earth complexes, and achieves the effects of high yield, simple operation and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
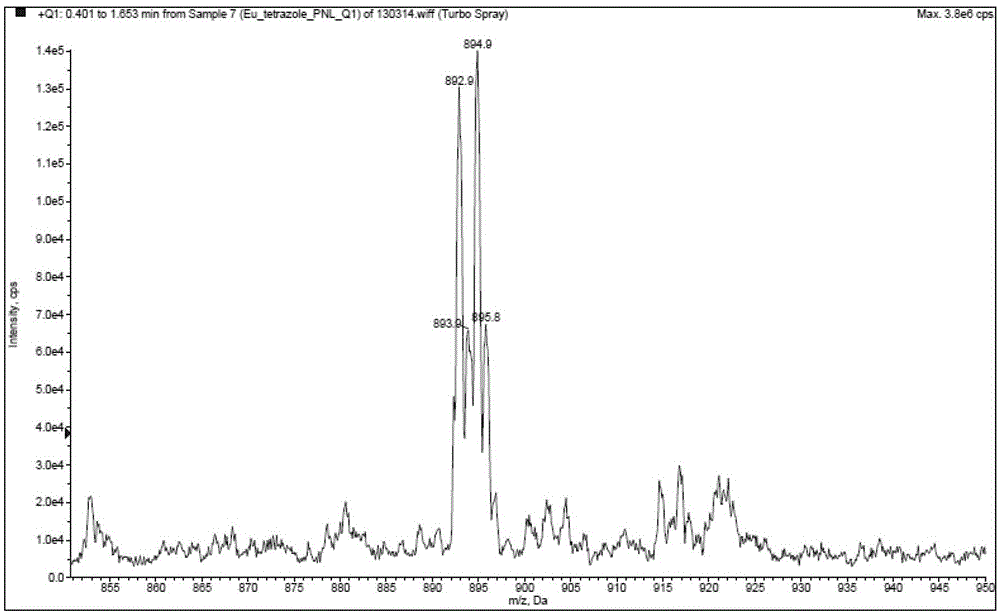
[0039] Example 1: Synthesis and fluorescence emission spectrum of tris[5-(2,2'-bipyridin-6-yl)-1,2,3,4-1H-tetrazole]europium(III) (compound 5) test
[0040] Step 1: Preparation of N-oxy-2,2’-bipyridine (compound 2)
[0041]
[0042] First, compound (1) 2,2’-bipyridine (100g) and acetic acid (500mL) were added into a 2L three-neck flask, stirred evenly, and 30% H 2 o 2 (70mL), heated to 70-75°C, stirred for 3 hours, cooled to room temperature, added 30% H 2 o 2 (70mL), continue heating to 60-80°C, and react for 3 hours. After cooling to room temperature, acetic acid was concentrated under reduced pressure to obtain a reddish-brown viscous oil, which was diluted with water (1000 mL), and the pH was adjusted to 8-9 with solid sodium carbonate. The resulting solution was extracted with dichloromethane (1000 mL+500 mL×3), and the organic phases were combined and dried with anhydrous sulfuric acid. After filtration, the filtrate was concentrated under pressure to obtain a r...
Embodiment 2
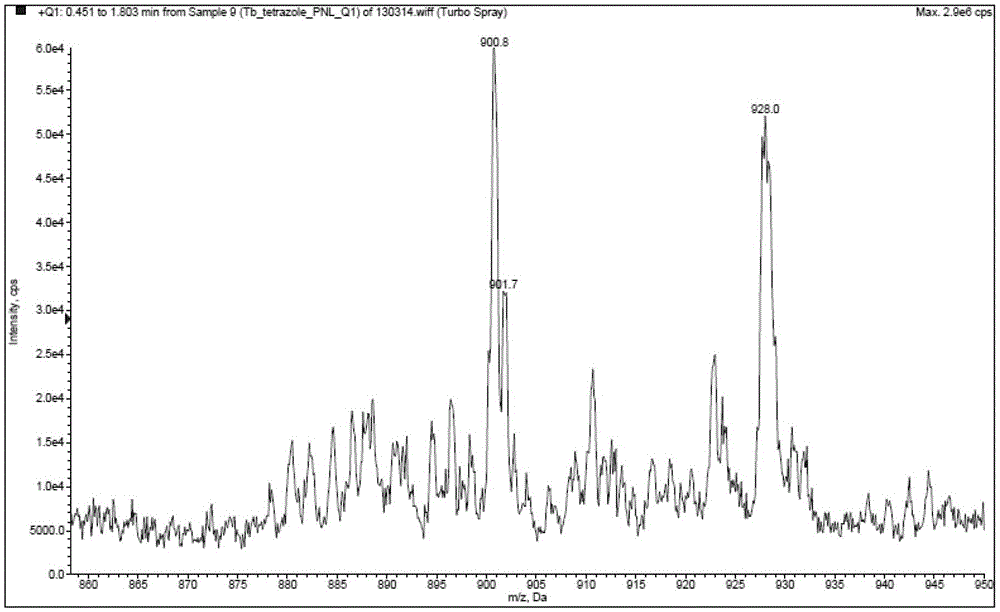
[0055] Example 2: Synthesis and fluorescence emission spectrum of tris[5-(2,2'-bipyridin-6-yl)-1,2,3,4-1H-tetrazole]terbium(III) (compound 6) test
[0056]
[0057] Compound 4 (7.4 g) and terbium trichloride hexahydrate (3.7 g) obtained in the previous example were dissolved in 50 mL of a mixed solvent of absolute ethanol and water at a volume ratio of 1:3 to form solutions C and D. Add 1.2g of sodium hydroxide to solution C, and stir for half an hour. Then, the D solution was added dropwise into the reaction bottle of the C solution, and the reaction was stirred at room temperature for 8 hours. After the reaction, the solvent was evaporated to dryness under reduced pressure, and the solid was vacuum-dried at 50° C. for 3 hours to obtain 9.3 g of yellow powder.
[0058] MS: [M+1] 828.9, TbC 33 h 21 N 18 M.W.=828, the ratio of M+H peak 829 and 830 peak height is detected close to the isotopic abundance ratio of Tb 2:1.
[0059] It can be known from the fluorescence emi...
Embodiment 3
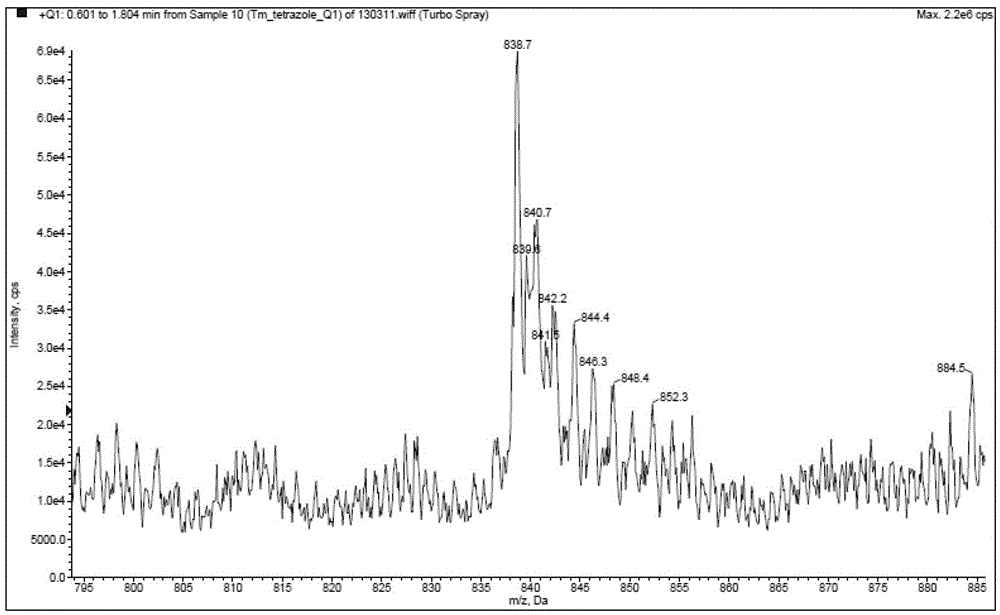
[0060] Example 3: Preparation and fluorescence emission spectrum of tris[5-(2,2'-bipyridin-6-yl)-1,2,3,4-1H-tetrazole]thulium(III) (compound 7) test
[0061]
[0062] Compound 4 (6.7g) obtained in Example 1 and thulium nitrate (3.5g) were respectively dissolved in 50mL of a mixed solvent of 1,2-propanediol and water with a volume ratio of 1:3 to form solutions E and F. Add 1.2g of sodium hydroxide into solution E, and stir for half an hour. Then, the F solution was added dropwise into the reaction bottle of the E solution, and stirred and reacted for 48 hours under the condition of 60° C. water bath. After the reaction, the solvent was evaporated to dryness under reduced pressure, and the solid was dried in vacuum at 50° C. for 4 hours to obtain 9.1 g of yellow powder.
[0063] MS: [M+1] 838.7, TmC 33 h 21 N 18 M.W.=838, the height ratio of M+H peaks 839 and 841 was detected in accordance with the isotopic abundance ratio of Tm. Mass spectrum such as figure 1 shown. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com