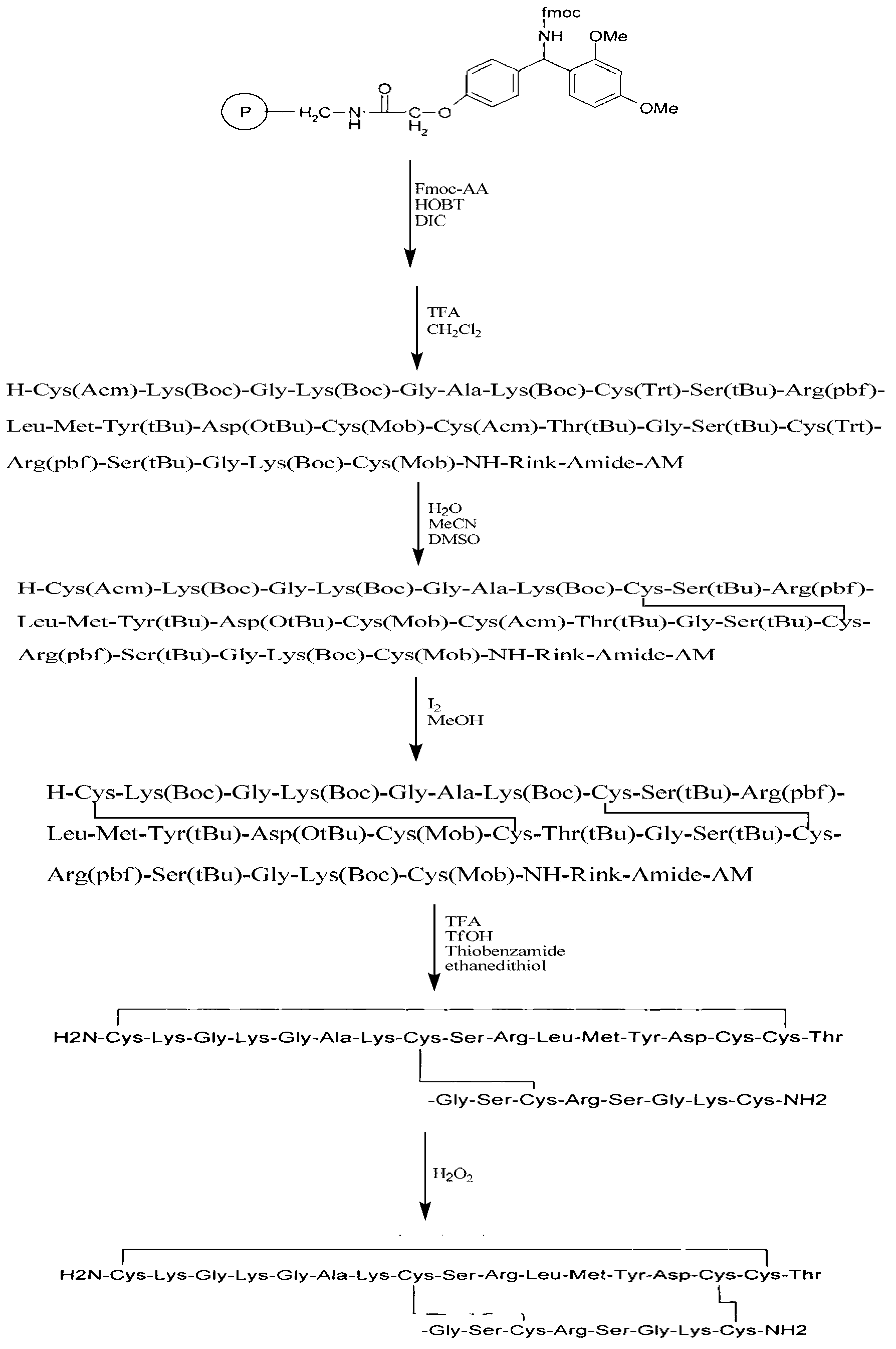
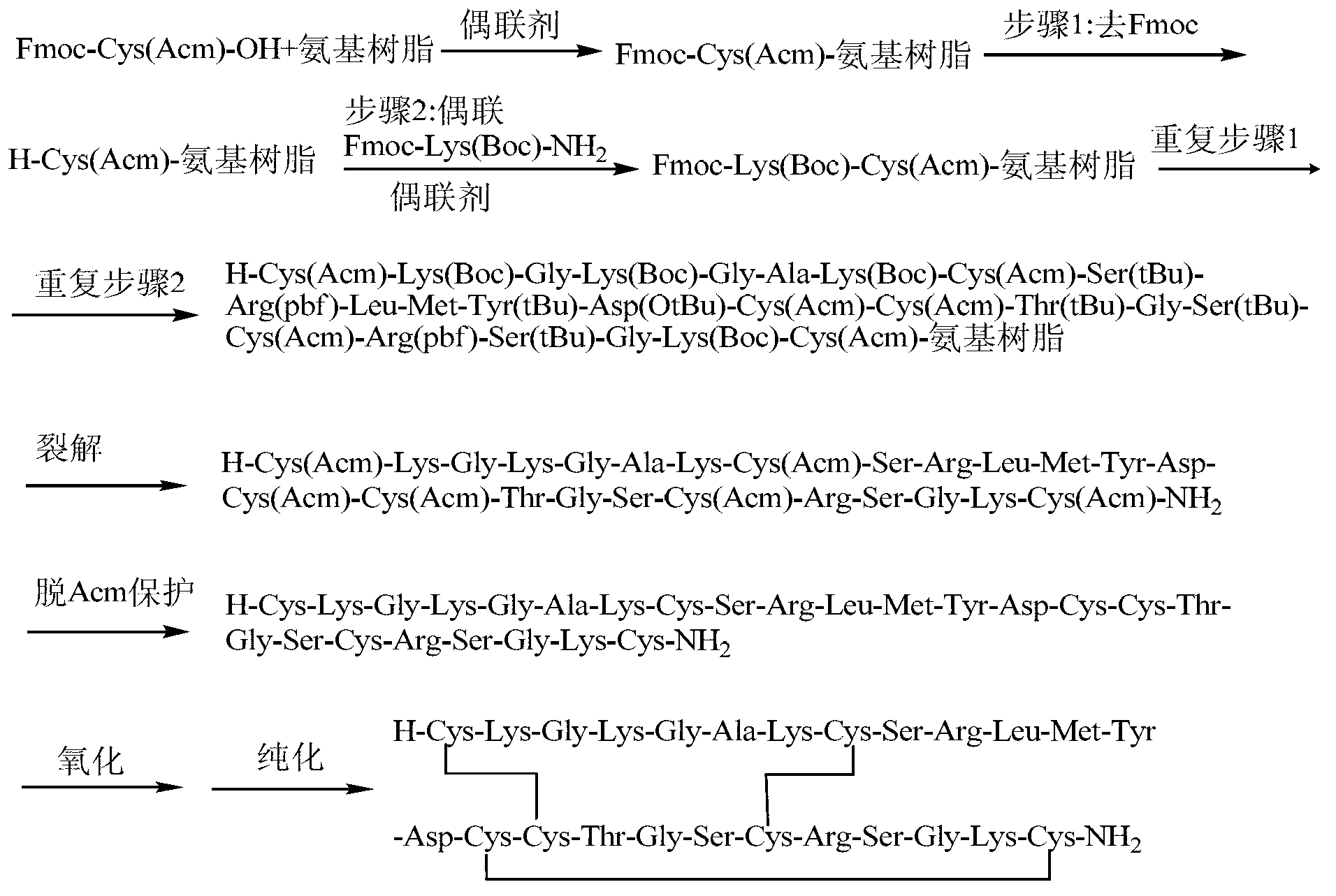
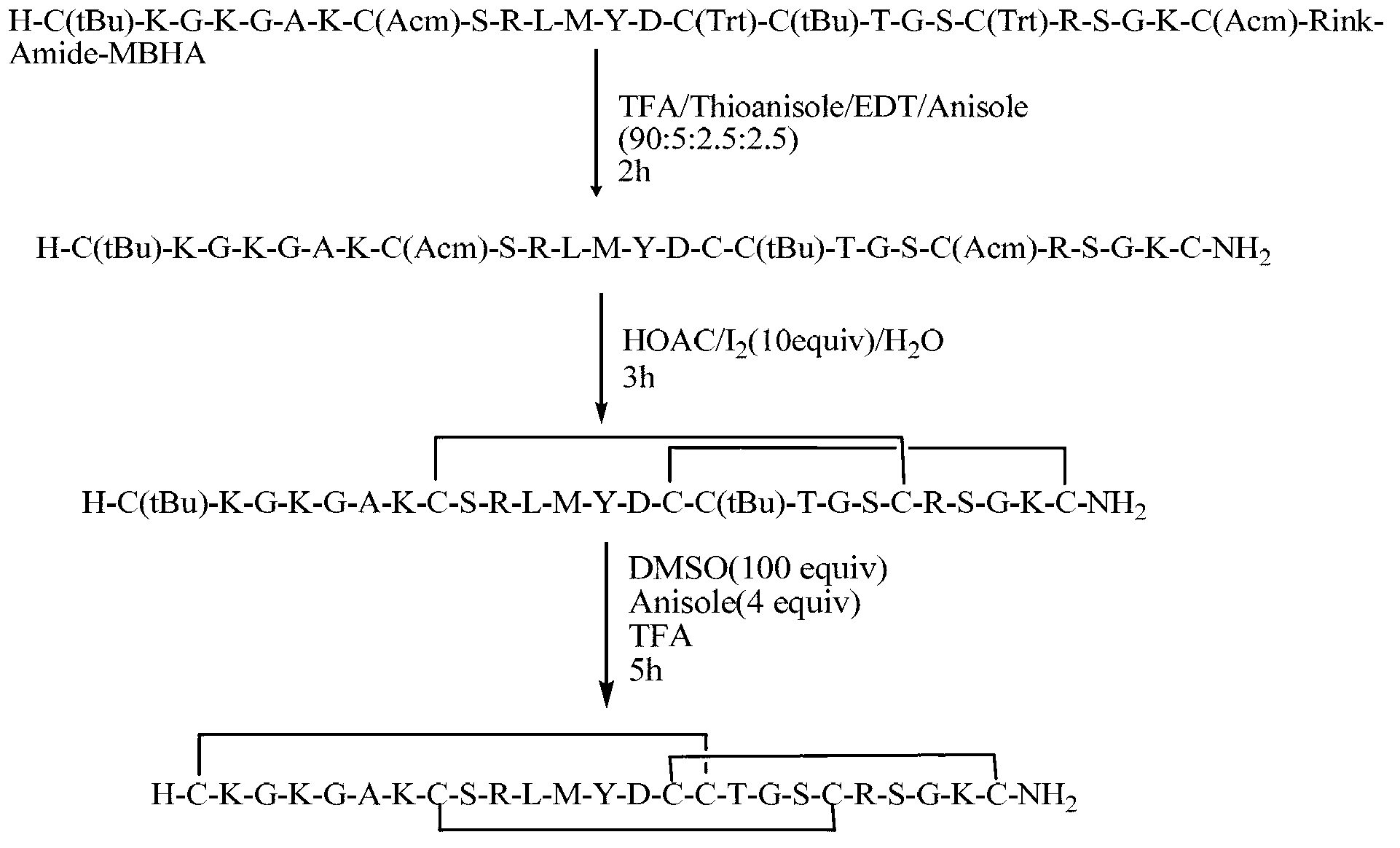
Solid-phase synthesis method of Ziconotide
A solid-phase synthesis, ziconotide technology, applied in the field of biochemistry, can solve the problems of low oxidation intensity of dimethyl sulfoxide and anisole, difficult to repeat tricyclic peptides, long reaction time, etc. Economical and practical value, easy preparation and purification, single product effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of Ziconotide Linear Fully Protected Peptide Resin.
[0061] (1) Resin swelling: Weigh 0.5g of Fmoc-Rink Amide-MBHA resin (SD=0.5mmol / g, 0.25mmol), add it to a 20ml BD syringe with a sieve plate, and use dimethylformamide and dimethicone Chloromethane was alternately washed 2 times each, 5ml / time, 3 minutes / time, and drained. Add dichloromethane twice the volume of the resin, shake at 640r / min for about 2 hours, and remove the dichloromethane by suction filtration. Then add dimethylformamide to wash the resin 3 times, 5ml / time, 3 minutes / time, and drain.
[0062] (2) Removal of the Fmoc protecting group: 5 ml of 20% piperidine / DMF (v / v) solution was added to the reactor, stirred at room temperature for 5 minutes, and drained. Repeat twice for 5 minutes and 15 minutes respectively. The ninhydrin method was used to detect the removal of Fmoc. Then alternately wash with dimethylformamide and dichloromethane 3 times each, 5 ml / time, 3 minutes / time...
Embodiment 2
[0070] Example 2: Cleavage of Ziconotide Linear Fully Protected Peptide Resin.
[0071] (1) Add the linear fully protected peptide resin obtained in Example 1 into a 50ml round bottom flask, and configure cutting agent 1 (mix trifluoroacetic acid, phenol, water, and TIS according to the volume ratio of 88:5:5:2) or Cutting agent 2 (mix trifluoroacetic acid, thioanisole, ethanedithiol, and anisole in a volume ratio of 90:5:3:2), add 12ml (10ml / g) cutting agent to the round bottom flask, 720r / min shaking reaction for 2 hours.
[0072] (2) After the cutting reaction is completed, drop the cutting solution into 10 times the volume of glacial ether (-20°C), then add 2 to 3ml of cutting agent 1 or 2 to the reactor to clean the resin, drop it into glacial ether, and A large number of peptides were precipitated and left to settle for half an hour. Centrifuge at 8000r / min at 4°C for 15min. Discard the supernatant, re-add glacial ether, oscillate and ultrasonically dissolve, balance,...
Embodiment 3
[0073] Example 3: Preparation of Ziconotide Single Disulfide Ring Crude Peptide.
[0074] (1) Add 30 mg of the linear peptide obtained in Example 2 into a 100 ml round-bottomed flask, add 60 ml of 5% acetic acid aqueous solution to dissolve to a concentration of 0.5 mg / ml, adjust the pH to 6 with a saturated ammonium carbonate aqueous solution, add 15 ml of DMSO, 640 r / ml Min room temperature shaking reaction for 2 hours.
[0075] (2) After the reaction is over, add 150ml of 0.05% trifluoroacetic acid-5% acetonitrile solution to terminate the reaction. The solution was lyophilized to obtain 28.5 mg of crude ziconotide monodisulfide ring peptide, with a crude yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com