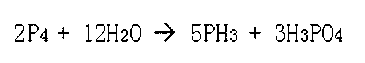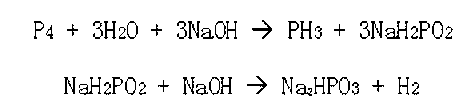Phosphine preparation method
A technology of phosphine and yellow phosphorus, which is applied in the direction of phosphine, etc., can solve the problems of increasing production costs and large amounts of phosphoric acid, and achieve the effects of reducing waste discharge, increasing yield, and reducing raw material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 2.0g of yellow phosphorus and 20g of methanol into the reaction flask, replace the air in the reaction flask with nitrogen and maintain the nitrogen flow, the pressure is slightly higher than atmospheric pressure; stir 4.0g of sodium hydroxide solution with a mass percentage concentration of 50% at 25°C While slowly dropping into the reaction flask, slowly raise the temperature to 60°C, the color of the reaction solution turns reddish brown, and phosphine is released. After the sodium hydroxide solution is added dropwise, continue to stir for 5 hours, and the reaction mixture changes from reddish brown to Earthy yellow, pH 9; add 5.2g of 36% hydrochloric acid to make the pH of the mixture 2; add 15g of absolute ethanol, stir at 50°C for 1 hour to obtain a beige liquid-solid mixture; filter the solid mixture at room temperature , the filter cake was washed with a small amount of absolute ethanol, and the filtrate was evaporated to dryness under reduced pressure to obt...
Embodiment 2
[0023] Add 2.0g of yellow phosphorus and 20g of ethanol into the reaction flask, replace the air in the reaction flask with nitrogen and maintain the nitrogen flow; slowly drop 4.0g of sodium hydroxide solution with a mass percentage concentration of 50% into the reaction flask while stirring at 25°C In the flask, slowly increase the temperature to 50°C, the color of the reaction solution turns reddish brown, phosphine is released, and after the sodium hydroxide solution is added dropwise, continue to stir for 4 hours, the reaction mixture changes from reddish brown to khaki, and the pH is 8 Add 2.6g of sulfuric acid with a concentration of 98% to make the pH of the mixture 1; add 15g of isopropanol and stir for 0.5 hours at 40°C to obtain a beige liquid-solid mixture; filter the solid mixture at room temperature, and filter the cake with a small amount of isopropanol Wash with propanol, and evaporate the filtrate to ethanol under reduced pressure to obtain 2.7 g of a viscous m...
Embodiment 3
[0025] Add 2.0g of yellow phosphorus and 20g of isopropanol into the reaction flask, replace the air in the reaction flask with nitrogen and maintain the nitrogen flow; slowly drop 4.0g of sodium hydroxide solution with a mass percentage concentration of 50% at 30°C while stirring Put it into the reaction flask, slowly increase the temperature to 60 ° C, the color of the reaction solution turns reddish brown, phosphine is released, and after the sodium hydroxide solution is added dropwise, continue to stir for 6 hours, the reaction mixture changes from reddish brown to khaki, and the pH 9; add 4.0 g of phosphoric acid with a concentration of 85% to make the pH of the mixture 1; add 15 g of isopropanol and stir at 50°C for 0.5 hours to obtain a beige liquid-solid mixture; filter the solid mixture at room temperature, and filter the cake with Wash with a small amount of isopropanol, and evaporate the filtrate to dryness under reduced pressure to obtain 3.0 g of a viscous mixed ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com