Pharmaceutical composition for preventing and/or treating diabetes mellitus
A technology of diabetes and composition, which is applied in the direction of drug combination, metabolic disease, medical formula, etc., can solve the problems of unstable quality control of traditional Chinese medicine, processing of crude preparations, unknown active ingredients, etc., and achieve the protection of islet cells, great application prospects, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of medicine for treating diabetes
[0045] The astragalus polysaccharide is prepared from astragalus root through conventional extraction process and spray drying, and the polysaccharide content is 50% after testing. The specific method is as follows: take 500 g of astragalus decoction pieces (Shenzhen Lifeng Pharmaceutical Company, batch number 111128), add 10 times the amount of distilled water to reflux for extraction for 2 hours, filter, add 10 times the amount of water to extract again for 1 hour, and filter. The two filtrates were combined and concentrated to 500ml, added 3 times the volume fraction of 95% ethanol solution, left standing overnight at low temperature, centrifuged, suction filtered, and vacuum-dried to obtain astragalus polysaccharide.
[0046] Hawthorn flavonoids are prepared from hawthorn fruit through conventional extraction process and spray drying, and the flavonoid content is 30% after testing. The specific metho...
Embodiment 2
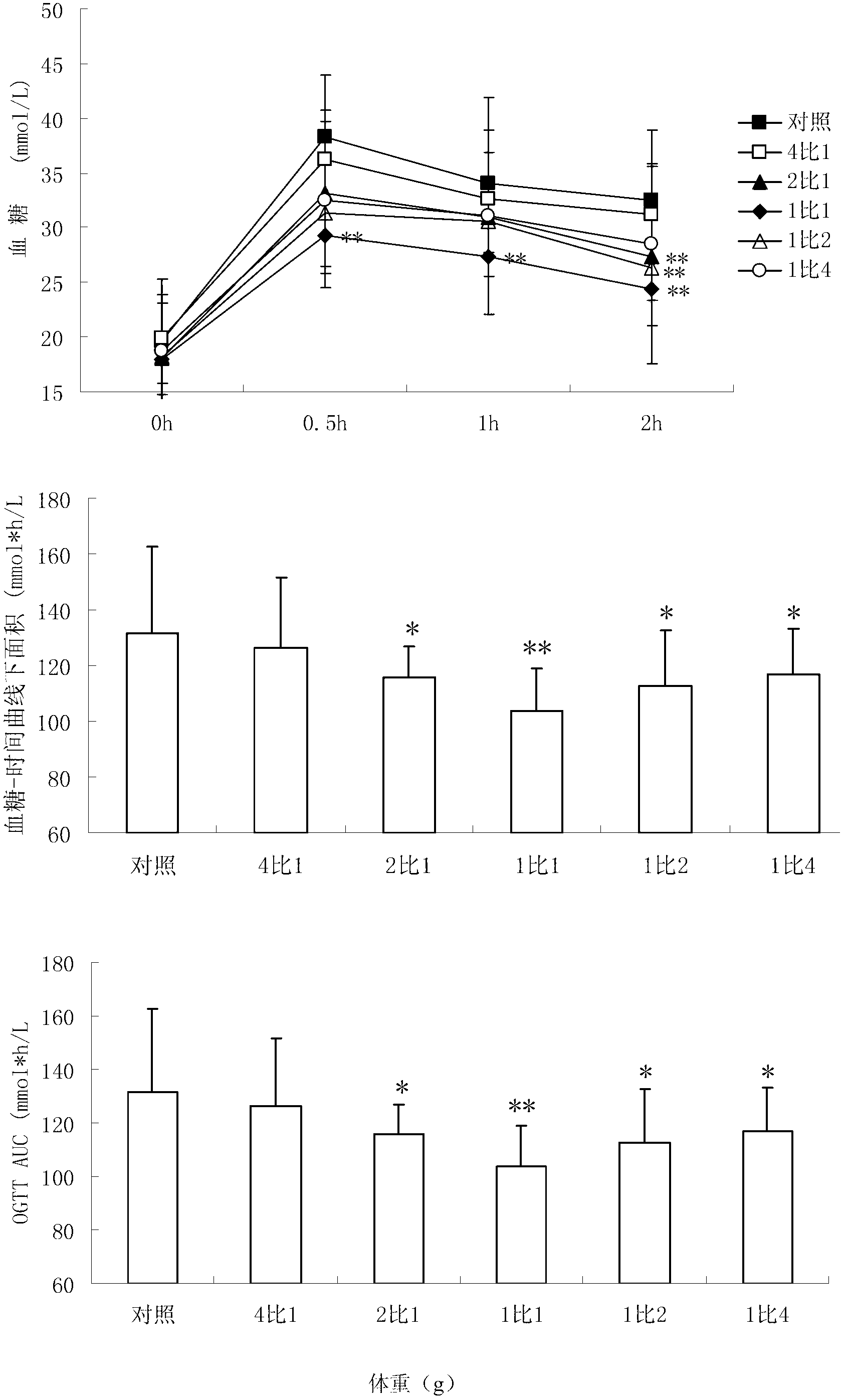
[0047] Embodiment 2, the influence of the drug ratio of different proportions on the oral glucose tolerance test of diabetic mice
[0048] 1. Production of NIH mouse diabetes model
[0049] NIH male mice (about 20 g) were fasted for 24 hours, and injected intraperitoneally once with 100 mg / kg streptozotocin (Streptozotocin, Sigma Company, USA) (streptozotocin was freshly prepared with ice-bathed citrate buffer solution in advance). reconstituted (0.1M, pH 4.5), injected within 5 minutes). After 10 days, the mice with a stable increase in blood sugar (blood sugar above 11.1 mmol / L) were the NIH mouse diabetes models.
[0050] 2. Oral glucose tolerance test
[0051] After overnight fasting of diabetic mice, 2.5g / kg glucose solution was administered into the stomach, and blood was collected from the eye sockets of the mice at time points 0, 0.5, 1 and 2 hours respectively, and the serum was centrifuged to detect the glucose concentration, and the blood glucose and The change i...
Embodiment 3
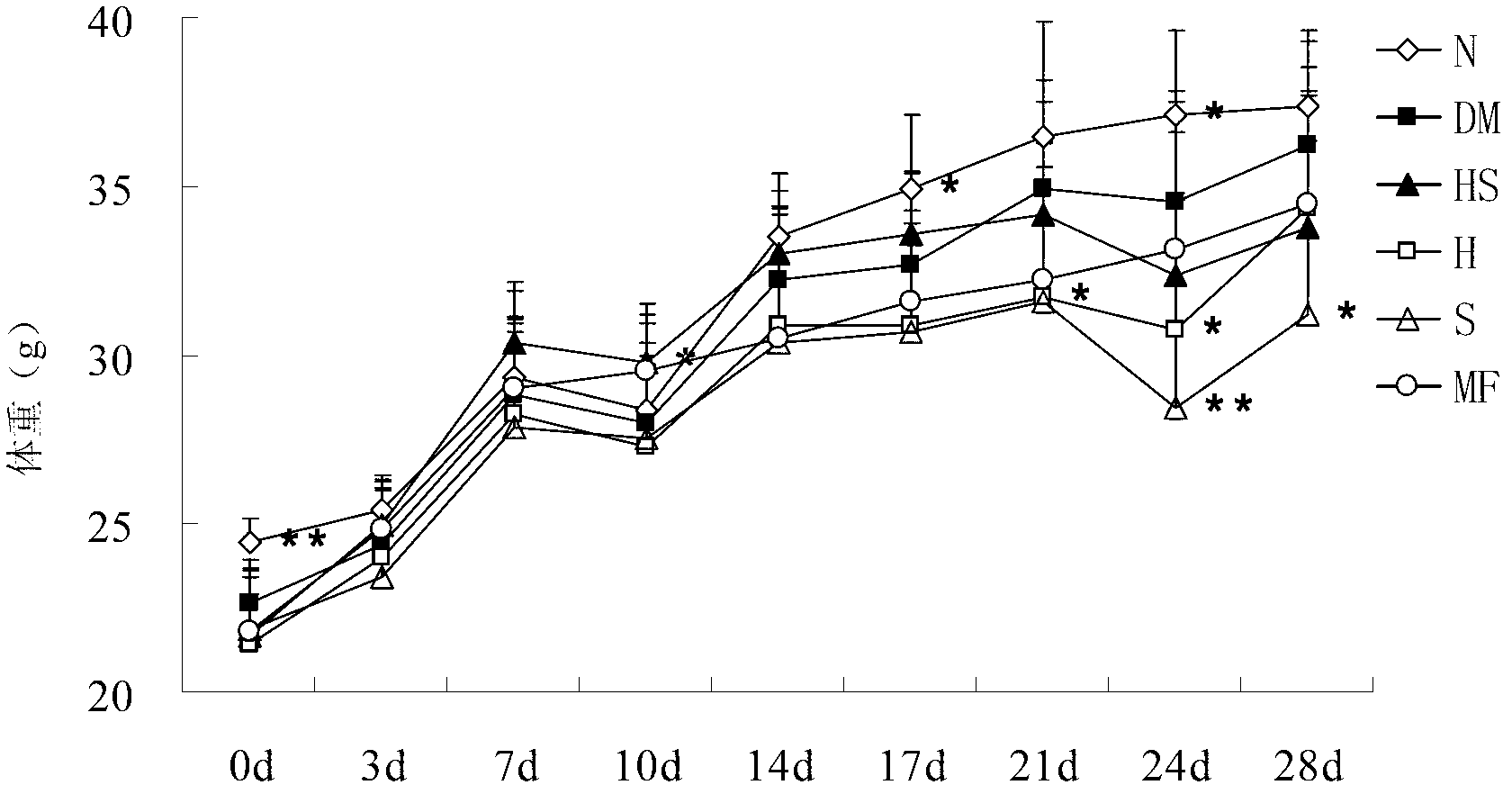
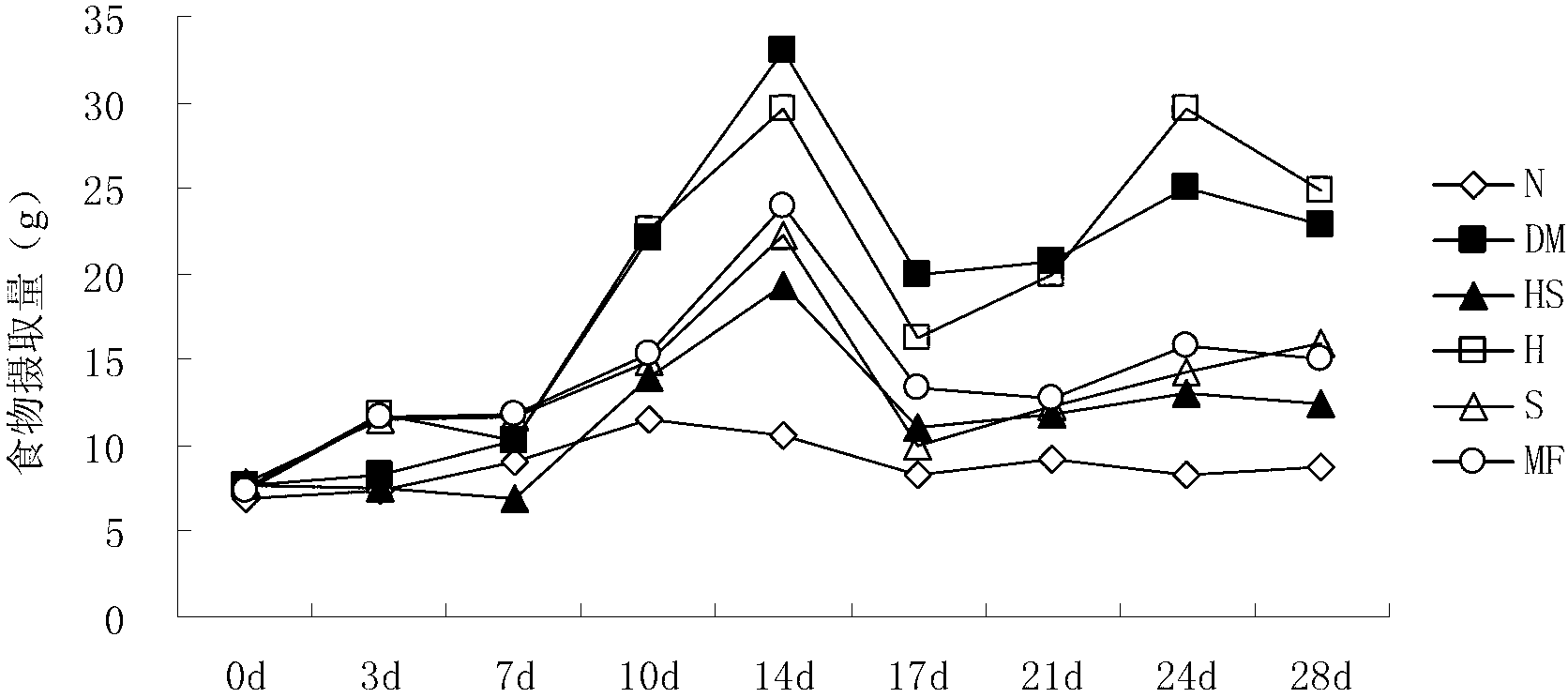
[0054] Embodiment 3, the drug effect of the medicine for treating diabetes
[0055] 1. Production of NIH mouse diabetes model
[0056] With embodiment 2.
[0057] 2. Administration of NIH mouse diabetes model
[0058] After successful screening of diabetes models, grouping (n=10) was carried out, and intragastric administration was carried out according to the following drug components for four consecutive weeks.
[0059] Astragalus polysaccharides and hawthorn flavonoids mixed treatment group (HS): In the NIH mouse diabetes model, astragalus polysaccharides and hawthorn flavonoids were mixed at a net content ratio of 1:1, and the net content was 200 mg / kg / day for intragastric administration. medicine.
[0060] Astragalus polysaccharide treatment group alone (H): In the NIH mouse diabetes model, astragalus polysaccharide was administered orally with a net content of 200 mg / kg / day.
[0061] Hawthorn flavonoids alone treatment group (S): In the NIH mouse diabetes model, hawt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com