Method for preparing hollow polymer microsphere coated with phase change material
A phase change material and hollow microsphere technology, which is applied in the field of preparation of polymer hollow microspheres, can solve the problems of thermal conductivity, mechanical properties of sealing performance, and insignificant improvement of thermal stability performance, and achieves large latent heat of phase change, High coverage and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
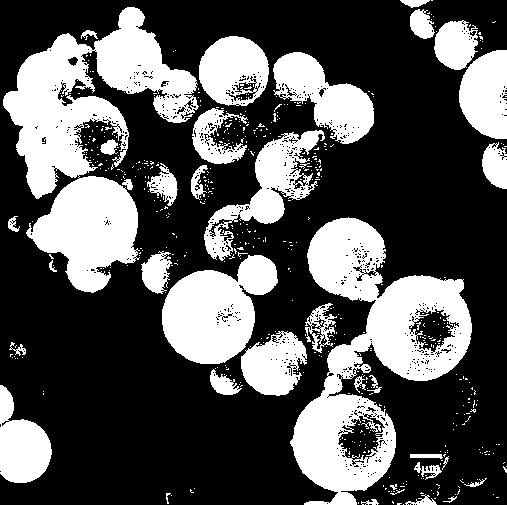
Image
Examples
Embodiment 1
[0026] 1. Uniformly disperse the ionic surfactant cetyltrimethylammonium bromide (CTAB) in water to prepare an aqueous solution with a mass fraction of 1%, and add it dropwise to a 6% hydrophilic solution with stirring at room temperature. Sex SiO 2 In aqueous solution of nanoparticles, SiO 2 The average particle size is 40nm. The weight of CTAB accounts for the SiO 2 The mass percentage is 10%, and the reaction is stirred for 1h to obtain the modified SiO 2 Nano Hydrosol.
[0027] 2. To the above modified SiO 2 Nano hydrosol, adding acrylonitrile monomer containing a carbon-carbon double bond, the weight of acrylonitrile is SiO 2 10 times the weight of nanoparticles; then add the phase change material dodecyl alcohol, the mass of dodecyl alcohol is SiO 2 10 times the weight of nanoparticles; then adding trimethylolpropane triacrylate containing three carbon-carbon double bonds, the weight of SiO 2 5 times the weight of the nanoparticles; finally add the initiator benzo...
Embodiment 2
[0030] 1. Experimental device and operation are the same as embodiment 1, silicon dioxide (SiO2) in embodiment 1 2 ) was changed to graphene oxide (GO), the average particle size was changed from 40nm to 2nm in thickness, and the width was 800nm, the mass fraction of aqueous solution of GO nanoparticles was changed to 0.2%, and the ionic surfactant CTAB was changed to hexadecylmethyl bromide Ammonium, the mass fraction of the surfactant aqueous solution was changed from 1% to 0.5%, and the mass percentage of the surfactant accounted for the mass percentage of GO nanoparticles was changed from 10% to 40%.
[0031] 2. The experimental device and operation are the same as in Example 1, and the monomer acrylonitrile in Example 1 is changed to styrene, and the quality of styrene monomer is 15 times that of the GO nanoparticle quality; trimethylolpropane triacrylate is changed to Divinylbenzene, the mass of divinylbenzene is 8 times the mass of GO nanoparticles; the phase change mat...
Embodiment 3
[0034] 1. Experimental device and operation are the same as embodiment 1, silicon dioxide (SiO2) in embodiment 1 2 ) to titanium dioxide (TiO 2 ), the average particle size is changed from 40nm to 10nm, TiO 2 The aqueous solution mass fraction of nanoparticle is changed into 1%, and ionic surfactant CTAB is changed into sodium dodecylbenzene sulfonate, and the mass fraction of surfactant aqueous solution 1% is changed into 2%, and the mass fraction of surfactant accounts for TiO 2 The mass percentage of nanoparticles was changed from 10% to 5%.
[0035] 2. experimental apparatus and operation are the same as embodiment 1, the monomer acrylonitrile in embodiment 1 is changed into the mixture of acrylonitrile and methyl acrylate, quality is TiO 2 20 times the mass of nanoparticles; trimethylolpropane triacrylate was changed to dipropylene glycol diacrylate, mass TiO 2 10 times the mass of nanoparticles; the phase change material lauryl alcohol is changed to octadecanoic acid,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com