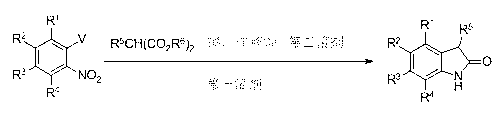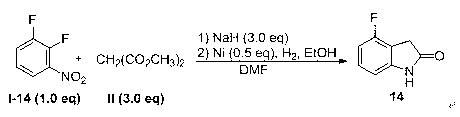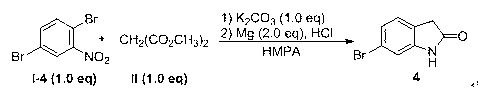One-pot series synthetic method of oxoindole
A technology of an indole oxide and a synthesis method, which is applied in the field of one-pot series synthesis of indole oxide, can solve the problems of difficulty in large-scale production, narrow substrate application range, narrow application substrate range and the like, and achieves convenient post-processing procedures. , Wide range of application, avoid the effect of solvents and reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The specific steps of the one-pot tandem synthesis method of the present invention comprise successively:
[0053] 1) Aromatic nucleophilic substitution reaction: add malonate, base MB, and the first solvent in sequence in the reaction bottle, heat to 25°C~60°C and stir, cool to room temperature, add o-nitrohalide containing substituents After substituting benzene, reheat to 25°C~160°C and stir until the reaction raw materials are completely converted;
[0054] 2) Krapcho deesterification reaction: After the above step 1) the aromatic nucleophilic substitution reaction is complete, heat to 110-180°C to remove the ester group until the deesterification reaction is completed;
[0055] 3) After the deesterification reaction in the above step 2) is completed, cool to room temperature, then add a second solvent such as acetic acid and a reducing agent, and heat to 25°C~150°C to react until TLC shows that the reaction is complete;
[0056] 4) The target product, oxidindole, ...
Embodiment 1
[0070] Oxindole 1 Synthesis:
[0071]
[0072] Dimethyl malonate (2.64 g, 20 mmol) was added to a mixture of potassium carbonate (2.76 g, 20 mmol) and 2.0 mL of anhydrous N, N-dimethylformamide, heated to 60 °C, and reacted for 20 After 1 min, cool to room temperature and add 2.0 mL o-chloronitrobenzene, reheat to 80°C, react for 2 h, follow the reaction process by TLC, when the conversion of raw materials is basically complete, directly heat to 160°C, follow the reaction by TLC, react for 20 After cooling to room temperature for h, 20 mL of acetic acid and iron powder (1.12 g, 20 mmol) were added directly, and then heated to 90°C for 1 h, followed by TLC until the reaction was complete. Stop the reaction, remove excess acetic acid under reduced pressure, add 20 mL of ethyl acetate to dissolve, wash the filtrate with 2 × 20 mL of 10% aqueous hydrochloric acid, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, perform silica gel column chromatogr...
Embodiment 2
[0074] 6-Chloroindole 2 Synthesis:
[0075]
[0076] Diethyl malonate (640 mg, 4.0 mmol) was added to a mixture of potassium carbonate (552 mg, 4.0 mmol) and 2.0 mL of anhydrous dimethyl sulfoxide, heated to 35 °C, reacted for 30 min and cooled to room temperature, then add 2, 5-dichloronitrobenzene (384 mg, 2.0 mmol), and then reheat to 45 ° C for 2 h, TLC to track the reaction progress, when the raw material is basically converted, directly heated to 120 ° C, TLC Follow the reaction, continue to react for 8 h, then cool to room temperature, then directly add 2.0 mL of hydrochloric acid aqueous solution (1.0 mol / L), zinc powder (130 mg, 2.0 mmol), reheat to 30 ° C for 1 h, TLC traces the reaction progress to The response is complete. Stop the reaction, add 20 mL of ethyl acetate to dissolve, wash the filtrate with 2 × 20 mL of 10% aqueous hydrochloric acid, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and obtain the product by silica gel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com