Compound gentamicin sulphate in-situ gel for injection and preparation method thereof
A technology of gentamicin sulfate and in-situ gel, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Taking medicine, time-consuming and troublesome problems, etc., to achieve good pharmacological effects, reduce the number of administrations, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
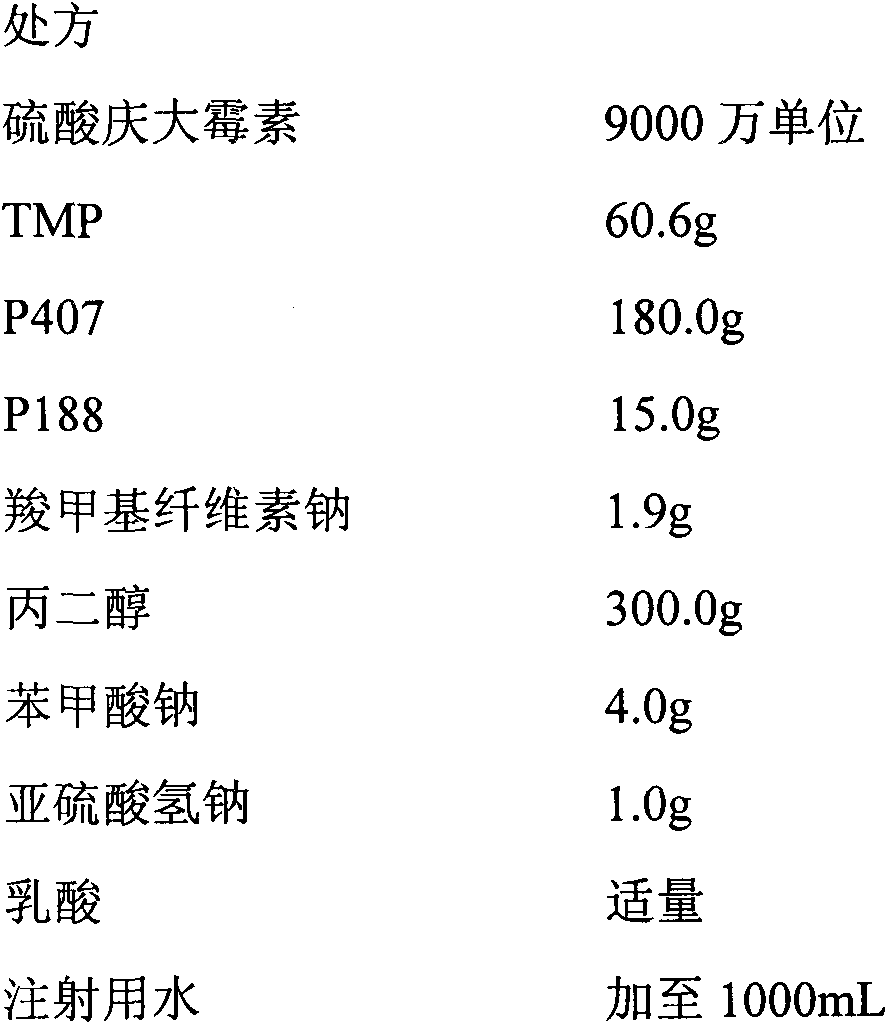
[0029] Compound gentamicin sulfate in situ gel for injection (each mL contains gentamicin 90,000 units, TMP60mg)
[0030]
[0031] Preparation method: Take propylene glycol, add appropriate amount of water for injection, heat to 50-60°C, add TMP, dissolve with appropriate amount of lactic acid, then add gentamicin sulfate, sodium benzoate, sodium bisulfite, stir to dissolve; the selected gel Sprinkle the matrix and sodium carboxymethyl cellulose on the above liquid surface, refrigerate at 4°C for more than 24 hours, until a clear, lump-free, uniformly dispersed solution is obtained, dilute to volume with water for injection, stir evenly, filter through micropores, and dispense .
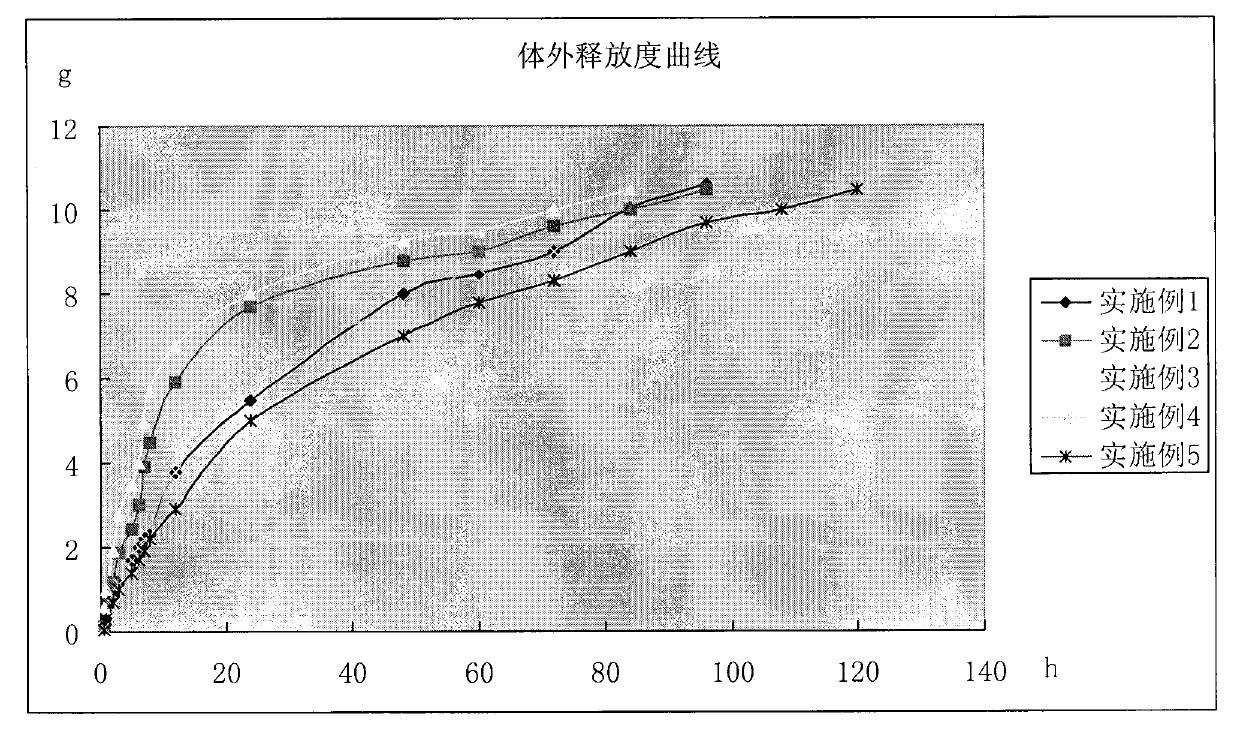
[0032] The prepared in situ gel was evaluated in vitro, including gelation temperature, gelation time, thermal reversibility, release rate, viscosity, pH value, etc. The measurement methods of each item are as follows, the measurement results are shown in Table 1, and the in vitro release results...
Embodiment 2
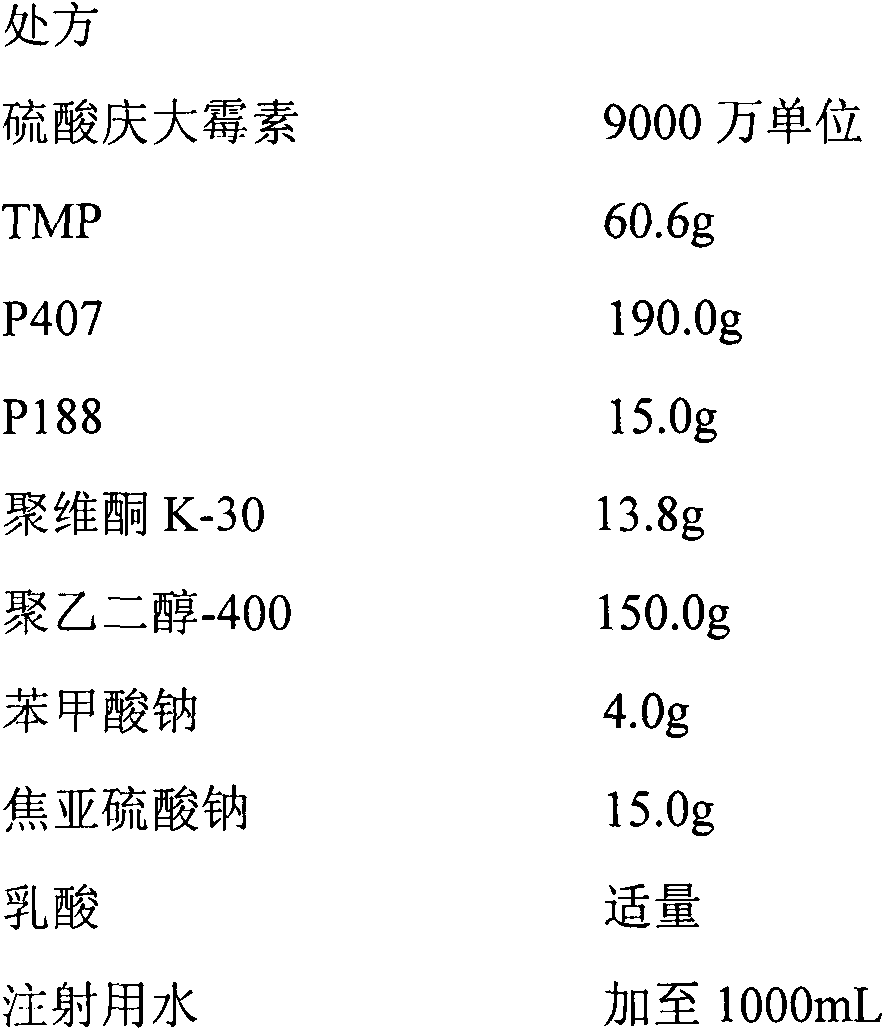
[0040] Compound gentamicin sulfate in situ gel for injection (each mL contains gentamicin 90,000 units, TMP60mg)
[0041]
[0042] Preparation method: Take propylene glycol, add appropriate amount of water for injection, heat to 50-60°C, add TMP, add appropriate amount of lactic acid to dissolve, add gentamicin sulfate, sodium benzoate, sodium metabisulfite, stir to dissolve; the selected gel matrix and poly Sprinkle vitamin K-30 on the above-mentioned liquid surface, refrigerate at 4°C for more than 24 hours, until a clear, lump-free, uniformly dispersed solution is obtained, dilute to volume with water for injection, stir evenly, filter through micropores, and divide into packages. The in vitro performance evaluation method is the same as that in Implementation 1. The results are shown in Table 1, and the in vitro release results are shown in the accompanying drawings.
Embodiment 3
[0044] Compound gentamicin sulfate in situ gel for injection (each mL contains gentamicin 90,000 units, TMP60mg)
[0045]
[0046]
[0047] Preparation method: Take propylene glycol, add appropriate amount of water for injection, heat to 50-60°C, add TMP, add appropriate amount of lactic acid to dissolve, add gentamicin sulfate, sodium benzoate, sodium pyrosulfite, stir to dissolve; sprinkle the selected gel base on On the above liquid surface, refrigerate at 4°C for more than 24 hours until a clear, lump-free, uniformly dispersed solution is obtained, dilute to volume with water for injection, stir evenly, filter through micropores, and divide into packages. The in vitro performance evaluation method is the same as that in Implementation 1. The results are shown in Table 1, and the in vitro release results are shown in the accompanying drawings.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com