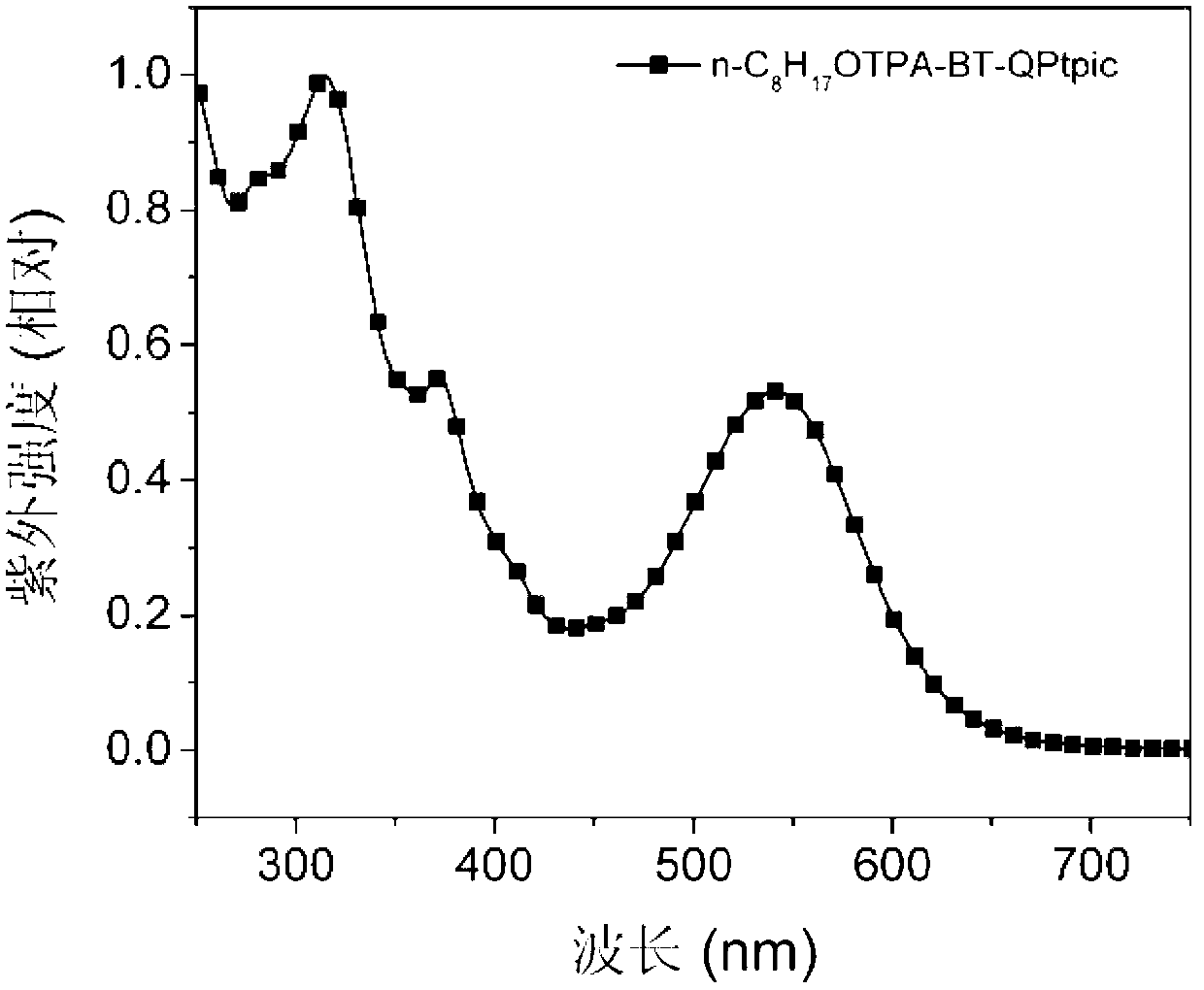
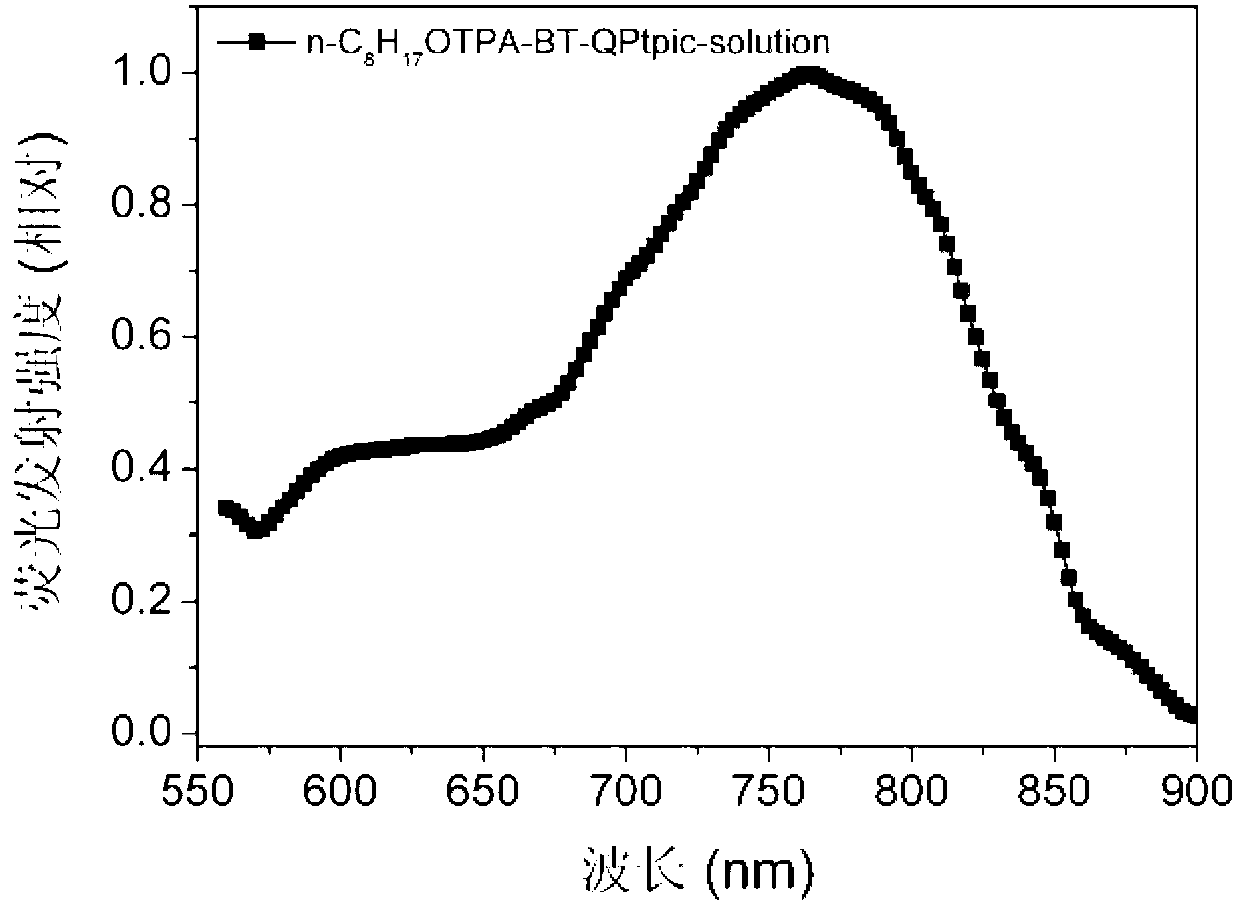
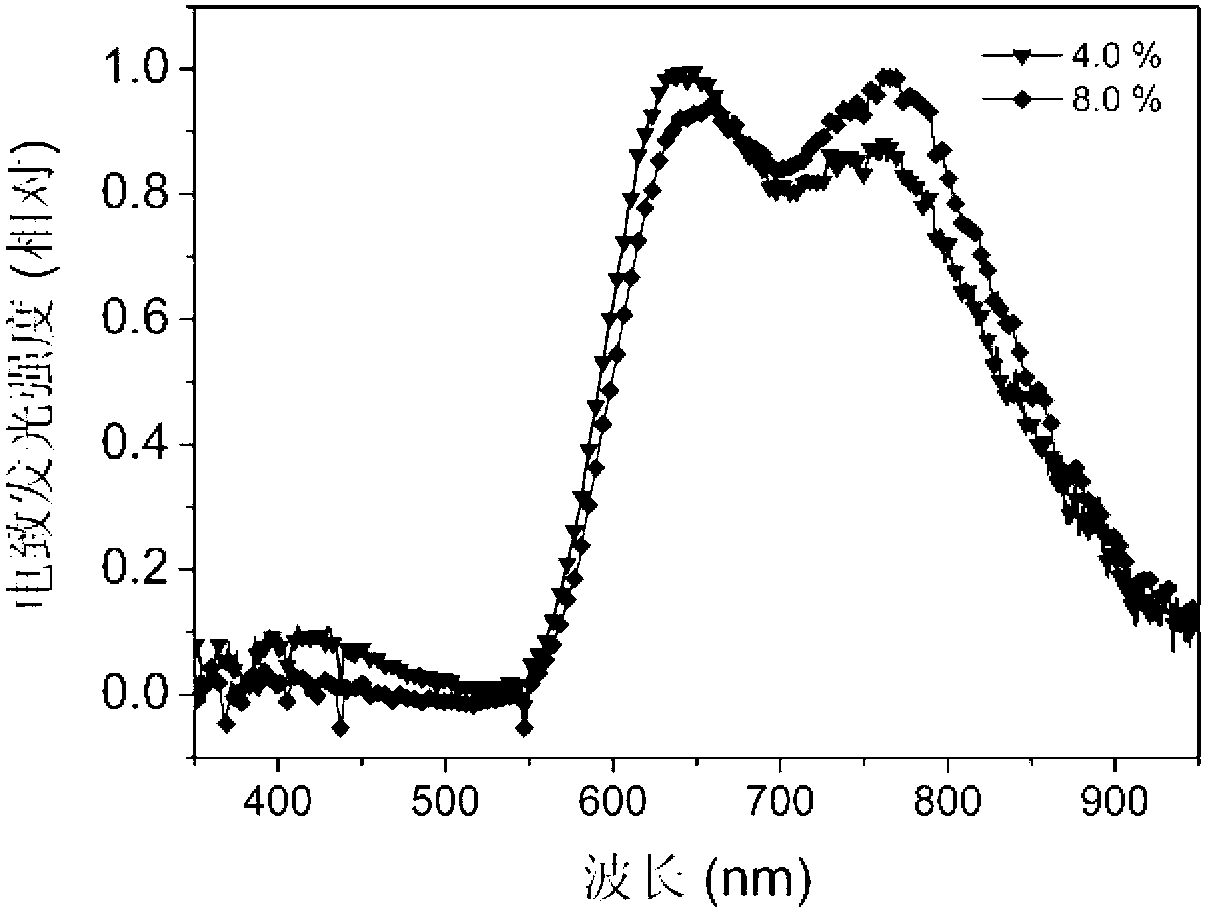
D-A-A type C<^>N ligand compound as well as C<^>N ring platinum complex and application thereof
A D-A-A, ligand compound technology, applied in the field of organic electroluminescence, can solve the problems of constraints, few types of infrared electroluminescent materials, and easy quenching of luminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] n-C 8 h 17 Synthesis of OTPA-BT-Q Ligand
[0061] 1.1 Synthesis of 4-n-octoxy iodobenzene
[0062] In a 250mL single-necked bottle, add 20.00g (90.91mmol) p-iodophenol, 26.34g (136.48mmol) n-octane bromide, 25.10g (180.58mmol) anhydrous potassium carbonate, 100mL acetone, and reflux for 24h under nitrogen protection. The reaction was stopped, and after cooling to room temperature, the reaction solution was poured into 150 mL of distilled water, extracted with dichloromethane (3×30 mL), and the organic layer was washed with anhydrous MgSO 4 dry. Filtration, rotary distillation under reduced pressure to remove the solvent, the obtained crude product was separated by 200-300 mesh silica gel column chromatography with petroleum ether as eluent to obtain 27.80 g of colorless transparent liquid with a yield of 92.1%. 1 H NMR (400MHz, CDCl 3 ,ppm):7.54(d,J=8.6Hz,2H),6.68(d,J=8.6Hz,2H),3.94-3.96(t,J=6.5Hz,2H),1.81-1.79(t,J= 4.2Hz,2H),1.46-1.16(m,10H),0.90-0.87(t,J=7.4Hz,3...
Embodiment 2
[0075] i-C 8 h 17 Synthesis of Cz-BT-Q Ligand
[0076] 2.1 Synthesis of 4-bromo-7-(9-isooctylcarbazol-2-yl)-2,1,3-benzothiadiazole
[0077] In a 100mL single-necked bottle, add 1.60g (3.95mmol) 2-(9-isooctyl) carbazole borate, 1.38g (4.74mmol) 4,7-dibromo-2,1,3-benzo Thiadiazole, 8 mL of 2M potassium carbonate aqueous solution, 8 mL of anhydrous methanol, 137 mg (0.12 mmol) of tetrakis(triphenylphosphine) palladium, 40 mL of toluene, reacted at 80° C. for 12 h under nitrogen protection. Stop the reaction, after cooling to room temperature, the reaction solution was poured into 150mL distilled water, extracted with dichloromethane (3 × 30mL), the organic layer was anhydrous MgSO 4 dry. Filtration, the solvent was distilled off under reduced pressure, and the residue was separated by 200-300 mesh silica gel column chromatography with dichloromethane:petroleum ether=1:5 (V / V) as the eluent to obtain 1.18g of a yellow viscous substance , and the yield was 61.2%. 1 H NMR (400...
Embodiment 3
[0083] 3.1 n-C 8 h 17 The synthesis of OTPA-BT-QPtpic complex, the structural formula is as follows:
[0084]
[0085] In a 50mL single-necked bottle, add 150mg (0.20mmol) n-C 8 h 17 OTPA-BT-Q ligand, 65 mg (0.16 mmol) potassium chloroplatinite (K 2 PtCl 4 ), 5 mL of distilled water and 15 mL of ethylene glycol monoethyl ether were reacted at 80° C. for 24 h under nitrogen protection. Cool to normal temperature, add 25mL distilled water, extract the mixture with dichloromethane (3×15mL), combine the organic phase with anhydrous MgSO 4 Dry, distill off the solvent under reduced pressure, and dry in a vacuum oven to obtain 140 mg of a purple-black solid, which is the chlorine bridged product (n-C 8 h 17 OTPA-BT-Q) 2 (μ-Cl) 2 Pt(II), with a yield of 90.7%, was directly used in the next step of de-bridging reaction.
[0086] In a 50mL single-necked bottle, add 140mg (0.07mmol) (n-C 8 h 17 OTPA-BT-Q) 2 (μ-Cl) 2 Pt(II), 34mg (0.28mmol) picolinic acid, 30mg (0.28mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com