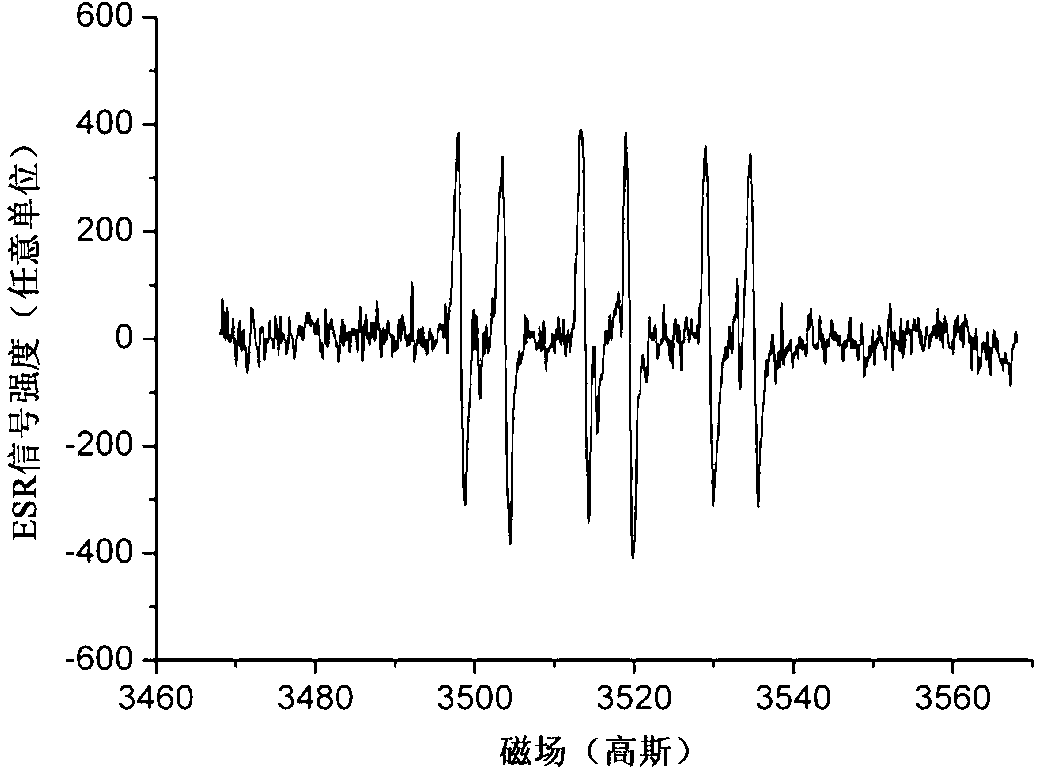
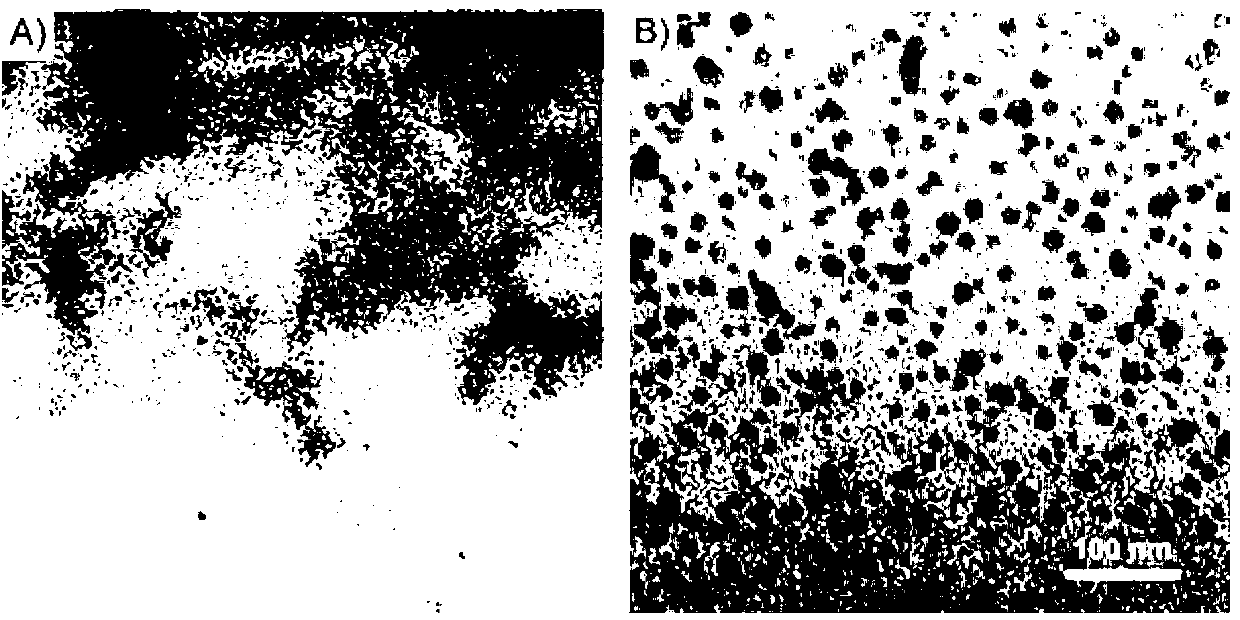
Method for preparing hydrogel by enzymatic free radical polymerization
A free radical and hydrogel technology, which is applied in the field of hydrogel preparation by enzymatic free radical polymerization, can solve the problems of complex product purification in the reaction process and poor mechanical strength of hydrogel, and achieve simple operation, mild reaction conditions, and environmental protection. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Preparation of vinyl-modified bovine serum albumin: Take 600 mg of bovine serum albumin (BSA) and 54 mg of N-acryloyloxysuccinimide, dissolve them in 30 mL of deionized water, and react with magnetic stirring at room temperature for 2.5 hours. The reaction mixture was transferred to a dialysis bag, dialyzed in deionized water for 3 days, and freeze-dried to obtain a white solid product which was vinyl-modified bovine serum albumin. According to the method reported in the literature (J.Biosci.Bioeng., 2005, 100, 551-555), it is measured that on average, about 4 double bonds are modified on each BSA molecule.
[0028] 2) Preparation of precursor solution: Take 0.1021g of N,N-dimethylacrylamide, 0.0608g of vinyl-modified bovine serum albumin, and 1.7162g of deionized water into the sample bottle, mix well with a vortex mixer, and measure the pH value is 6.0.
[0029] 3) Preparation of hydrogel: 7.784 mg of acetylacetone, 100 μL of concentrated horseradish peroxidase so...
Embodiment 2
[0031] 1) Referring to Example 1, vinyl-modified bovine serum albumin was prepared and a precursor solution was prepared.
[0032]2) Preparation of hydrogel: Add 14.60 mg of acetylacetone, 100 μL of horseradish peroxidase concentrated solution, and 6 μL of aqueous hydrogen peroxide solution to the above precursor solution in sequence (the molar concentration ratio of acetylacetone and hydrogen peroxide in the reaction system is 5. The molar concentration ratio of hydrogen peroxide and horseradish peroxidase was 280), mixed quickly, and left in a closed place to obtain a light yellow transparent hydrogel with a gelation time of 58 s.
Embodiment 3
[0034] 1) Referring to Example 1, vinyl-modified bovine serum albumin was prepared and a precursor solution was prepared.
[0035] 2) Preparation of hydrogel: 36.01 mg of acetylacetone, 100 μL of concentrated horseradish peroxidase solution, and 6 μL of aqueous hydrogen peroxide solution were sequentially added to the above precursor solution (the molar concentration of acetylacetone and hydrogen peroxide in the reaction system was 12 , the molar concentration ratio of hydrogen peroxide and horseradish peroxidase is 280), mixed quickly, and left in airtight to obtain light yellow transparent hydrogel, gelation time 20s.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com