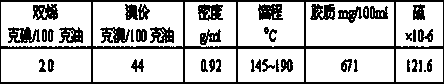Nickel base catalyst
A nickel-based catalyst, catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] With nitric acid in [H + ] / [AlOOH] molar ratio is under the condition of 0.25, with pseudo-boehmite powder peptization 24 hours, obtains the alumina sol 1500g that alumina solid content is 5%, then adds a certain amount of silica solid content and is 40% Silica sol, the two were mixed and stirred for 5 hours to obtain silica-alumina sol. Dissolve nickel chloride in an appropriate amount of water to obtain a nickel salt solution of 0.10 g nickel / ml. Add an appropriate amount of nickel salt solution to the silica-alumina sol, adjust the pH of the sol to 8.5 with ammonia water, and then o C aging for 24 hours, and then filtered and dried to obtain the corresponding catalyst precursor. catalyst precursor at 400 o C roasting for 4 hours to obtain oxidized NiO / Al 2 o 3 -SiO 2 catalyst. Catalyst in 1.5 L / min pure hydrogen flow, at 450 o Reduction at C for 12 hours to obtain metallic Ni / Al 2 o 3 -SiO 2 catalyst.
[0017] Change the amount of silica sol and nickel sa...
Embodiment 2
[0019] With nitric acid in [H + ] / [AlOOH] molar ratio is under the condition of 0.25, with pseudo-boehmite powder peptization 24 hours, obtains the alumina sol 1500g that alumina solid content is 5%, then adds a certain amount of silica solid content and is 40% Silica sol, the two were mixed and stirred for 5 hours to obtain silica-alumina sol. In terms of molar ratio, basic nickel carbonate: ammonia water: ammonium carbonate = 1: 6.0: 1.5, adding an appropriate amount of water to obtain a nickel-ammonia complex solution of 0.10 g nickel / ml. Add an appropriate amount of nickel-ammonia complex solution to the silica-alumina sol, at 95 o The complexed nickel ion is heated and decomposed at C for 8 hours, and then the corresponding catalyst precursor is obtained by filtering and drying. catalyst precursor at 400 o C roasting for 4 hours to obtain oxidized NiO / Al 2 o 3 -SiO 2 catalyst. Catalyst in 1.5 L / min pure hydrogen flow, at 450 o Reduction at C for 12 hours to obtain...
Embodiment 3
[0027] Aromatics account for 65 to 80% of the cracked C9 and above hydrocarbon fractions, and contain a large amount of polymerizable unsaturated components. This test example uses the cracked C9 and above hydrocarbons and saturated hydrogenated oil in a certain ratio. The raw materials (see Table 4 for specific components) were tested for the hydrogenation activity of the catalyst of the present invention. The catalyzer that embodiment 1~2 and comparative example 1 obtains at 450 o C was reduced with hydrogen for 24 hours. The hydrogenation reaction uses an adiabatic fixed-bed reactor, and the process conditions are: the inlet temperature is 50 o C, pressure 3.0 MPa, fresh oil space velocity LHSV=1.5 hours -1 , the volume ratio of hydrogen to oil H 2 / raw oil = 600:1, the experimental results are shown in Table 5.
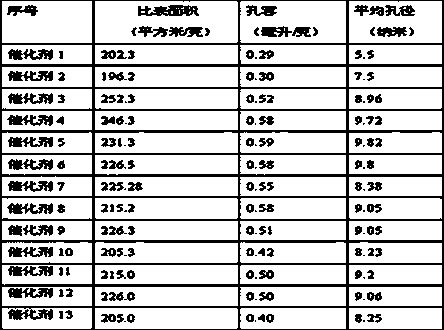
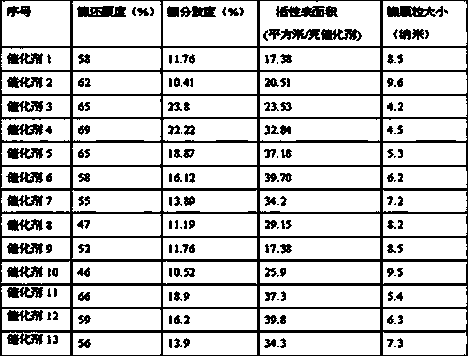
[0028] Table 2
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com