Method of preparing small molecule polyol from carbohydrate under near-critical or supercritical conditions
A technology of carbohydrates and supercritical water, which is applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., to achieve the effects of high space-time conversion rate, simple preparation process, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
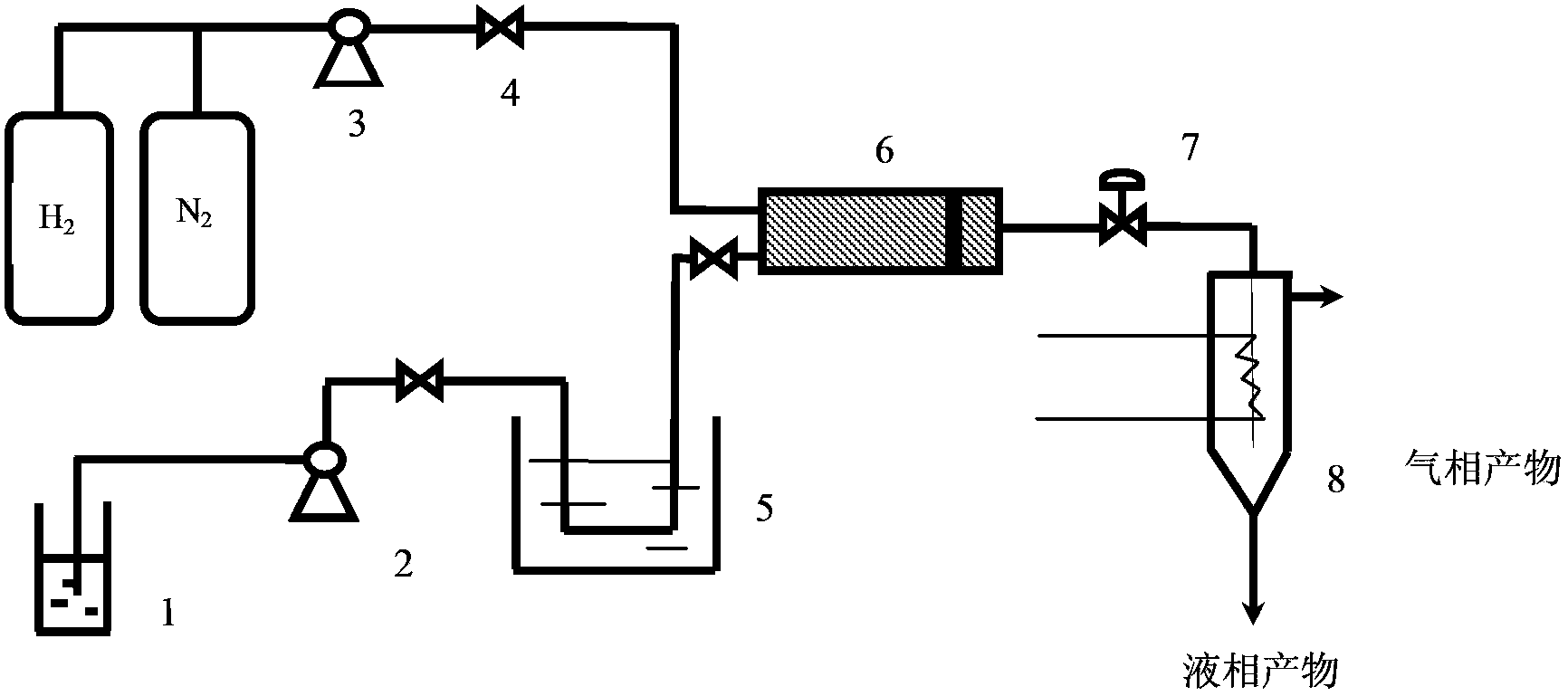
Image
Examples
Embodiment 1
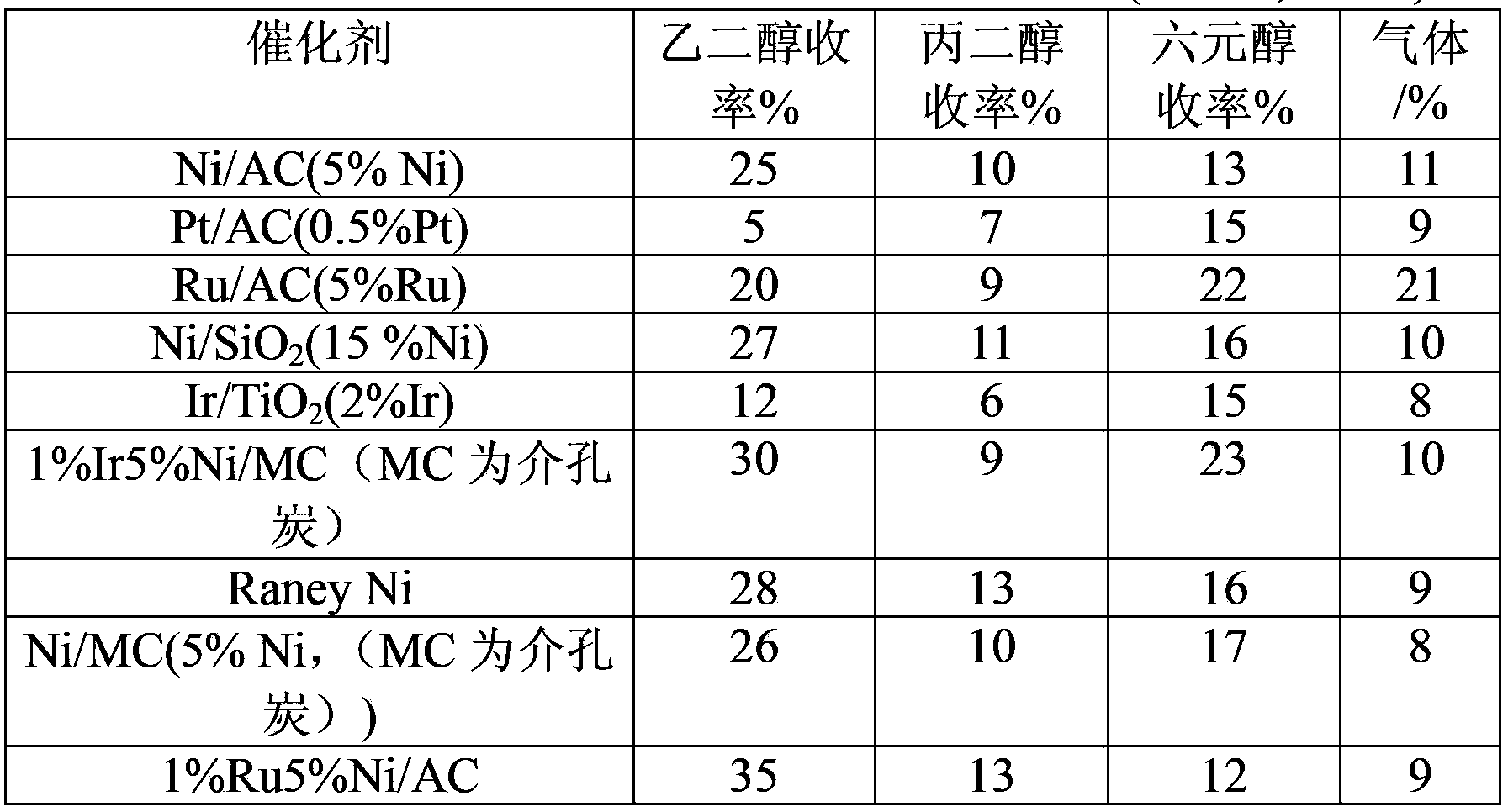
[0022] Metal catalyst Ni / AC,Ni / SiO 2 ,Pt / AC,Ru / AC,Ir / TiO 2 Preparation: impregnate nickel nitrate, chloroplatinic acid, ruthenium trichloride, and chloroiridic acid aqueous solution on the carrier, dry at 120°C for 12h, and then reduce it in a hydrogen atmosphere at 450°C for 1h to obtain the catalyst Ni / AC (5wt%Ni), Ni / MC(5wt%Ni), Pt / AC(0.5wt%Pt), Ru / AC(5wt%Ru), Ni / SiO 2 (15wt%Ni), Ir / TiO 2 (2wt%Ir) catalyst.
Embodiment 2
[0024] Preparation of bimetallic catalyst composed of noble metal and nickel:
[0025] Co-impregnation or step-by-step impregnation is used. Taking co-impregnation as an example, the specific operation steps are: take 1.0g of mesoporous carbon (MC) and add it to a chloride solution containing 1% Ir (Ru, Pt, Pd, Rh are all available, Ir is used as an example here) and In the 5ml aqueous solution of 0.259g nickel nitrate, stand by room temperature for 12 hours, then dry in the oven of 60 ℃ and 120 ℃ respectively for 12 hours. The as-prepared precursors were reduced at 450 °C for 1 h under a hydrogen atmosphere. The theoretical loading amount of nickel in the prepared catalyst is 5wt%, and the theoretical loading amount of noble metal is 1%. The final prepared catalyst is labeled as 1%Ir-5%Ni / MC.
Embodiment 3
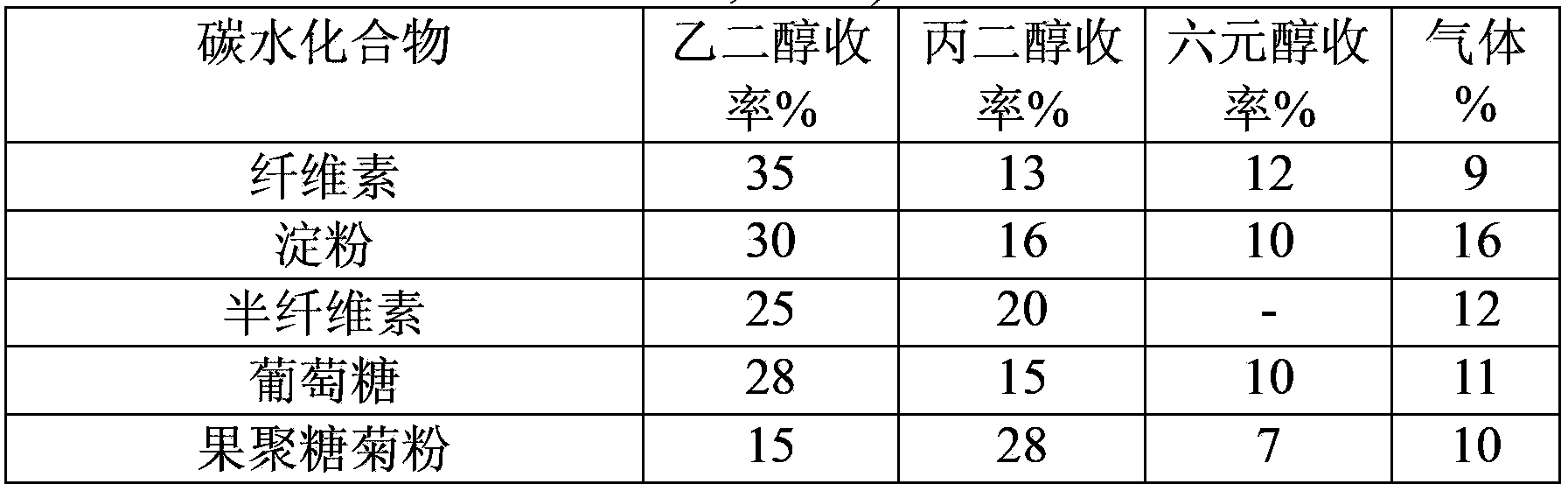
[0027] Catalytic conversion experiment: Add 0.5g of carbohydrates, 0.2g of catalyst and 50ml of water into a 100ml reactor, pass through hydrogen to replace the gas three times, fill it with hydrogen to 4MPa, place it in a tin bath at 355°C, and react for 5min. After the reaction is finished, cool down to room temperature, take the centrifuged supernatant, separate it on a high-performance liquid chromatography calcium-type ion-exchange column and detect it with a differential refraction detector. Ethylene glycol, propylene glycol, and hexahydric alcohols (including sorbitol, mannitol) are calculated in the product yield, and the gaseous product is CO 2 , CH 4 , C 2 h 6 Wait for the gas to calculate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com