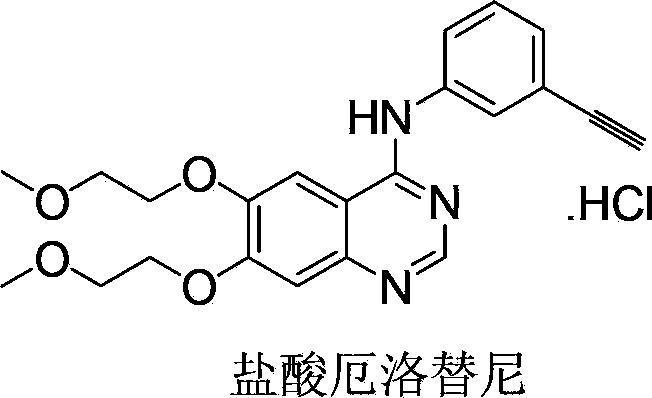
Industrialized production method for erlotinib hydrochloride B type crystal
A technology of erlotinib hydrochloride and erlotinib, which is applied in the field of producing erlotinib hydrochloride B-type crystal, can solve the problems of expensive acetonitrile, environmental pollution, and affecting the total yield, and achieves simple method and high yield , The effect of the method is simple and easy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 1. The preparation method of raw material Erlotinib is:
[0037] Type I crystal, type II crystal and type III crystal of erlotinib are prepared according to the method of CN101547910A; type G1 crystal, type G2 crystal, type G3 crystal and amorphous type of erlotinib are prepared according to the method of US2009012295.
[0038] 2. HPLC detection conditions are:
[0039] Chromatographic column: Agilent Eclipse XDB C8 (4.6×150mm×5μm), mobile phase: acetonitrile: 0.05mol / L potassium dihydrogen phosphate (42:58), injection volume: 20μl, column temperature: 30°C, detection wavelength: 247nm.
[0040] 3. X-powder diffraction conditions are:
[0041] Scanning instrument: German BRUKER D8 DISCOVER X-ray diffractometer, scanning conditions: CuKα radiation, tube voltage 40KV, tube current 40mA, scanning range 5~60 degrees. The scanning step is 0.02 degrees, and the scanning speed is 2.4 degrees / minute.
[0042] 4. The concentration of hydrochloric acid is the mass concentratio...
Embodiment 1
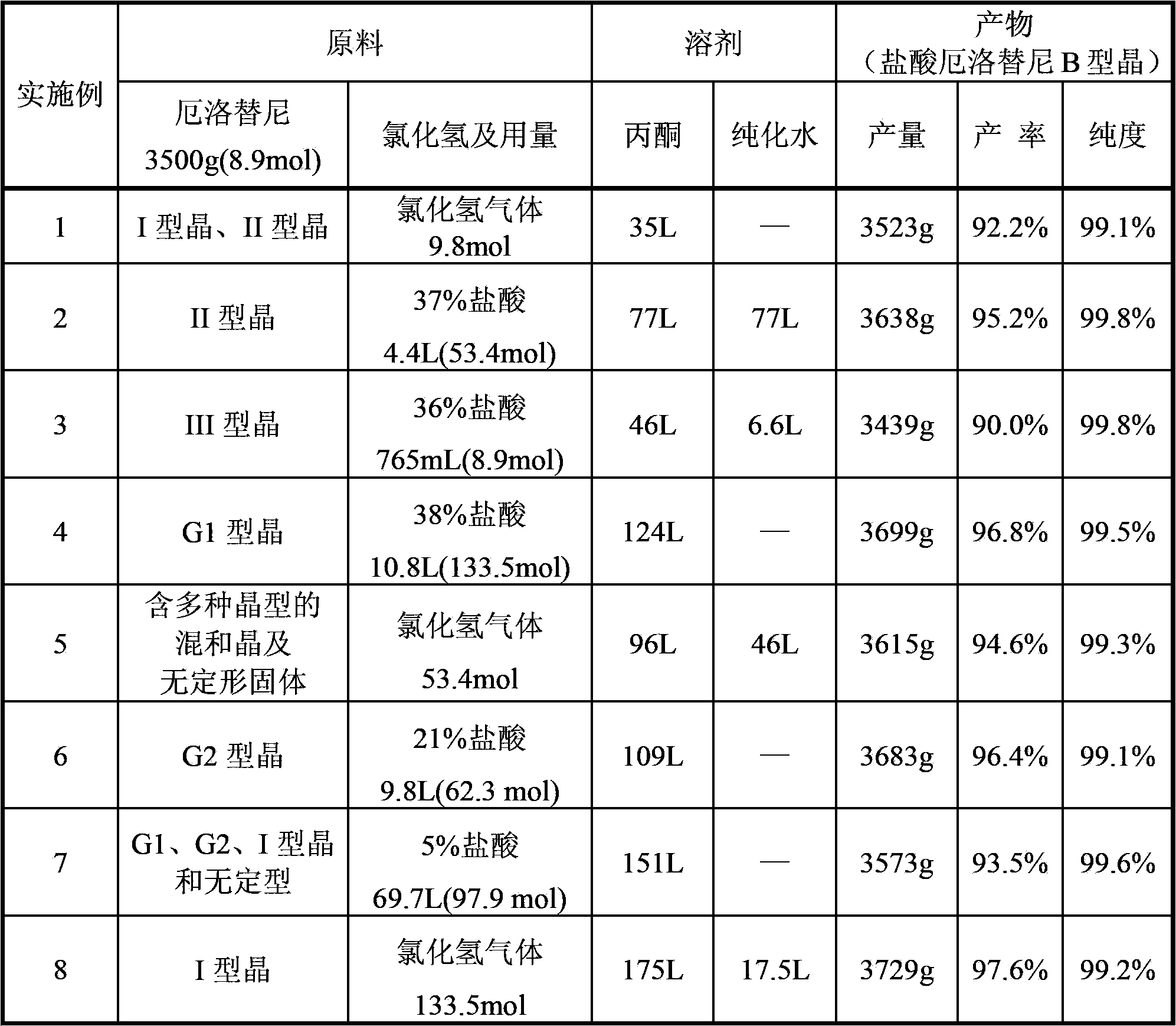
[0043] Example 1 Preparation of Erlotinib Hydrochloride Type B Crystal 393.17 with Erlotinib Mixed Crystal
[0044] Add 3500g of erlotinib mixed crystals (obtained according to the method of CN1066142C Example 20) and 35L of acetone into the reaction kettle at room temperature, start stirring, and heat in a water bath to completely dissolve erlotinib. Add 9.8 mol of hydrogen chloride gas to the solution.
[0045] After passing through, the reaction system was left to cool down slowly, and a large amount of solids were precipitated. After the reaction system no longer precipitates solids, centrifuge and dry under reduced pressure to obtain 3523g of finished product, the yield is 92.2%, the HPLC content is 99.1%, and the 2θ values measured by X-powder diffraction are: 6.259, 12.527, 13.402, 16.997, 20.224, 21.127 , 22.990, 24.493, 25.168, 26.923 (type B crystal).
Embodiment 2
[0046] Example 2 Preparation of Erlotinib Hydrochloride Type B Crystal with Erlotinib Type II Crystal
[0047] Add 3500g of erlotinib type II crystal, 77L of acetone, and 77L of purified water into the reaction kettle at room temperature, start stirring, and heat in a water bath to completely dissolve erlotinib. Add 4.4 L of 37% hydrochloric acid and continue stirring for 40 minutes.
[0048] The temperature of the reaction system was slowly lowered with a cold water bath. After a large amount of solids were precipitated, the temperature was then lowered with an ice water bath until no solids were precipitated in the reaction system. After centrifugation and drying under reduced pressure, 3638g of the finished product was obtained, the yield was 95.2%, the HPLC content was 99.8%, and the 2θ values measured by X-powder diffraction were: 6.250, 12.516, 13.381, 16.987, 20.223, 21.187, 22.972, 24.482, 25.226, 26.915 ( B-type crystal).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com